Abstract
Rational
The ghrelinergic system is implicated in the development of obesity and in modulating central reward systems. It has been reported that diet-induced obesity causes blunted responding on food intake to ghrelin administration, associated with central ghrelin resistance. Here we investigate whether the stimulatory effects of ghrelin on the reward system are altered in diet-induced obese mice.
Methods
Obesity was induced in C57BL/6J mice by feeding high-fat diet for 13 weeks. Mice were trained in an operant fixed and exponential progressive ratio task to respond for sucrose rewards. In an ad libitum fed state, ghrelin and a ghrelin receptor antagonist were administered in the progressive ratio. Alterations in the central ghrelin system in diet-induced obese mice were assessed.
Results
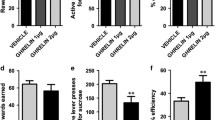
Obese mice showed attenuated acquisition and performance in the fixed and progressive ratio paradigm. Most importantly, diet-induced obesity inhibited the stimulatory effects of ghrelin (2 nmol, 3 nmol/10 g) on progressive ratio responding whereas lean animals presented with increased responding. Administration of the ghrelin-receptor antagonist (D-Lys3)-GHRP-6 (66.6 nmol/10 g) decreased performance in lean but not obese mice. This insensitivity to ghrelin receptor ligands in mice on high-fat diet was further supported by decreased mRNA expression of the ghrelin receptor in the hypothalamus and the nucleus accumbens in obese mice.
Conclusions
This study demonstrates that the modulatory effects of ghrelin receptor ligands are blunted in a mouse model of diet-induced obesity in a progressive ratio task. Thereby, our data extend the previously described ghrelin resistance in these mice from food intake to reward-associated behaviours.





Similar content being viewed by others
References
Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschöp MH, Gao XB, Horvath TL (2006) Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 116:3229–3239
Arnold JM, Roberts DC (1997) A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav 57:441–447
Asakawa A, Inui A, Kaga T, Katsuura G, Fujimiya M, Fujino MA, Kasuga M (2003) Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 52:947–952
Beck B, Richy S, Stricker-Krongrad A (2004) Feeding response to ghrelin agonist and antagonist in lean and obese Zucker rats. Life Sci 76:473–478
Briggs DI, Andrews ZB (2011) Metabolic status regulates ghrelin function on energy homeostasis. Neuroendocrinology 93:48–57
Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB (2010) Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology 151:4745–4755
Cheng KC, Li YX, Asakawa A, Inui A (2010) The role of ghrelin in energy homeostasis and its potential clinical relevance. Int J Mol Med 26:771–778
Davis KW, Wellman PJ, Clifford PS (2007) Augmented cocaine conditioned place preference in rats pretreated with systemic ghrelin. Regul Pept 140:148–152
Davis JF, Tracy AL, Schurdak JD, Tschöp MH, Lipton JW, Clegg DJ, Benoit SC (2008) Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci 122:1257–1263
Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E (2011) The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol 340(1):80–87
Egecioglu E, Jerlhag E, Salomé N, Skibicka KP, Haage D, Bohlooly-Y M, Andersson D, Bjursell M, Perrissoud D, Engel JA, Dickson SL (2010) Ghrelin increases intake of rewarding food in rodents. Addict Biol 15:304–311
Egecioglu E, Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Jerlhag E, Engel JA, Dickson SL (2011) Hedonic and incentive signals for body weight control. Rev Endocr Metab Disord 12(3):141–151
El-Ghundi M, O’Dowd BF, Erclik M, George SR (2003) Attenuation of sucrose reinforcement in dopamine D1 receptor deficient mice. Eur J Neurosci 17:851–862
Field BC, Chaudhri OB, Bloom SR (2009) Obesity treatment: novel peripheral targets. Br J Clin Pharmacol 68:830–843
Finger BC, Dinan TG, Cryan JF (2010) Progressive Ratio Responding in an Obese Mouse Model: Effects of Fenfluramine. Neuropharmacology 59:619–626
Finger BC, Dinan TG, Cryan JF (2011a) Behavioural satiety sequence in a genetic mouse model of obesity: effects of ghrelin receptor ligands. Behav Pharmacol. doi:10.1097/FBP.0b013e32834afee6
Finger BC, Dinan TG, Cryan JF (2011b) High-fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience. doi:10.1016/j.neuroscience.2011.06.072
Finger BC, Schellekens H, Dinan TG, Cryan JF (2011c) Is there altered sensitivity to ghrelin receptor ligands in leptin deficient mice?: Importance of satiety state and time of day. Psychopharmacology (Berl) 216:421–429
Haney M, Rubin E, Foltin RW (2011) Aripiprazole maintenance increases smoked cocaine self-administration in humans. Psychopharmacology (Berl) 216(3):379–387
Hodos W (1961) Progressive ratio as a measure of reward strength. Science 134:943–944
Jerlhag E, Engel JA (2011) Ghrelin receptor antagonism attenuates nicotine-induced locomotor stimulation, accumbal dopamine release and conditioned place preference in mice. Drug Alcohol Depend 117(2–3):126–131
Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA (2006) Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol 11:45–54
Jerlhag E, Egecioglu E, Dickson SL, Svensson L, Engel JA (2008) Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors are involved in mediating the ghrelin-induced locomotor stimulation and dopamine overflow in nucleus accumbens. Eur Neuropsychopharmacol 18:508–518
Jerlhag E, Egecioglu E, Landgren S, Salomé N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, Engel JA (2009) Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci USA 106:11318–11323
Jerlhag E, Egecioglu E, Dickson SL, Engel JA (2010) Ghrelin receptor antagonism attenuates cocaine- and amphetamine-induced locomotor stimulation, accumbal dopamine release, and conditioned place preference. Psychopharmacology (Berl) 211:415–422
Kenny PJ (2011) Reward mechanisms in obesity: new insights and future directions. Neuron 69:664–679
Kirchner H, Tong J, Tschöp MH, Pfluger PT (2010) Ghrelin and PYY in the regulation of energy balance and metabolism: lessons from mouse mutants. Am J Physiol Endocrinol Metab 298:E909–E919
Kitazawa T, De Smet B, Verbeke K, Depoortere I, Peeters TL (2005) Gastric motor effects of peptide and non-peptide ghrelin agonists in mice in vivo and in vitro. Gut 54:1078–1084
Landgren S, Simms JA, Hyytiä P, Engel JA, Bartlett SE, Jerlhag E (2011a) Ghrelin receptor (GHS-R1A) antagonism suppresses both operant alcohol self-administration and high alcohol consumption in rats. Addict Biol. doi:10.1111/j.1369-1600.2010.00280.x
Landgren S, Simms JA, Thelle DS, Strandhagen E, Bartlett SE, Engel JA, Jerlhag E (2011b) The ghrelin signalling system is involved in the consumption of sweets. PLoS One 6:e18170
Lutter M, Nestler EJ (2009) Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr 139:629–632
Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E (2000) Comparison of the effects of clozapine, haloperidol, chlorpromazine and d-amphetamine on performance on a time-constrained progressive ratio schedule and on locomotor behaviour in the rat. Psychopharmacology (Berl) 152:47–54
Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, Woloszyn J, Yanagisawa M, Lutter M, Zigman JM (2010) Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry 67:880–886
Pulman KJ, Fry WM, Cottrell GT, Ferguson AV (2006) The subfornical organ: a central target for circulating feeding signals. J Neurosci 26:2022–2030
Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11
Rickard JF, Body S, Zhang Z, Bradshaw CM, Szabadi E (2009) Effect of reinforcer magnitude on performance maintained by progressive-ratio schedules. J Exp Anal Behav 91:75–87
Roane HS (2008) On the applied use of progressive-ratio schedules of reinforcement. J Appl Behav Anal 41:155–161
Rodgers RJ, Holch P, Tallett AJ (2010) Behavioural satiety sequence (BSS): Separating wheat from chaff in the behavioural pharmacology of appetite. Pharmacol Biochem Behav 97:3–14
Sabino V, Cottone P, Blasio A, Iyer MR, Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP (2011) Activation of σ-Receptors Induces Binge-like Drinking in Sardinian Alcohol-Preferring Rats. Neuropsychopharmacology 36:1207–1218
Schellekens H, Dinan TG, Cryan JF (2010) Lean mean fat reducing “ghrelin” machine: hypothalamic ghrelin and ghrelin receptors as therapeutic targets in obesity. Review. Neuropharmacology 58:2–16
Schiöth HB, Muceniece R, Wikberg JE (1997) Characterization of the binding of MSH-B, HB-228, GHRP-6 and 153N-6 to the human melanocortin receptor subtypes. Neuropeptides 31:565–571
Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL (2011a) Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience 180:129–137
Skibicka KP, Hansson C, Egecioglu E, Dickson SL (2011b) Role of ghrelin in food reward: impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addict Biol. doi:10.1111/j.1369-1600.2010.00294.x
Sofuoglu M, Mooney M, Kosten T, Waters A, Hashimoto K (2011) Minocycline attenuates subjective rewarding effects of dextroamphetamine in humans. Psychopharmacology (Berl) 213:61–68
Stoops WW (2008) Reinforcing effects of stimulants in humans: sensitivity of progressive-ratio schedules. Exp Clin Psychopharmacol 16:503–512
Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR (2010) Intranasal cocaine functions as reinforcer on a progressive ratio schedule in humans. Eur J Pharmacol 644:101–105
Takahashi N, Patel HR, Qi Y, Dushay J, Ahima RS (2002) Divergent effects of leptin in mice susceptible or resistant to obesity. Horm Metab Res 34:691–697
Temple JL, Legierski CM, Giacomelli AM, Salvy SJ, Epstein LH (2008) Overweight children find food more reinforcing and consume more energy than do nonoverweight children. Am J Clin Nutr 87:1121–1127
Tessari M, Catalano A, Pellitteri M, Di Francesco C, Marini F, Gerrard PA, Heidbreder CA, Melotto S (2007) Correlation between serum ghrelin levels and cocaine-seeking behaviour triggered by cocaine-associated conditioned stimuli in rats. Addict Biol 12:22–29
Tschöp M, Heiman ML (2001) Rodent obesity models: an overview. Exp Clin Endocrinol Diabetes 109:307–319
Vlachou S, Markou A (2010) GABAB receptors in reward processes. Adv Pharmacol 58:315–371
Volkow ND, O’Brien CP (2007) Issues for DSM-V: should obesity be included as a brain disorder? Am J Psychiatry 164:708–710
Volkow ND, Wise RA (2005) How can drug addiction help us understand obesity? Nat Neurosci 8:555–560
Volkow ND, Wang GJ, Fowler JS, Telang F (2008) Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci 363:3191–3200
Vucetic Z, Reyes TM (2010) Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdiscip Rev Syst Biol Med 2:577–593
Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS (2001) Brain dopamine and obesity. Lancet 357:354–357
Wellman PJ, Davis KW, Nation JR (2005) Augmentation of cocaine hyperactivity in rats by systemic ghrelin. Regul Pept 125:151–154
Yi CX, Heppner K, Tschöp MH (2011) Ghrelin in eating disorders. Mol Cell Endocrinol 340(1):29–34
Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J (2004) Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci USA 101:10434–10439
Acknowledgments
The work described herein was supported by Enterprise Ireland under Grant Number CC20080001. JFC and TGD are also supported in part by Science Foundation Ireland in the form of a centre grant (Alimentary Pharmabiotic Centre). The centre is also funded by GlaxoSmithKline. JFC is funded by European Community’s Seventh Framework Programme; Grant Number: FP7/2007-2013, Grant Agreement 201714.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Finger, B.C., Dinan, T.G. & Cryan, J.F. Diet-induced obesity blunts the behavioural effects of ghrelin: studies in a mouse-progressive ratio task. Psychopharmacology 220, 173–181 (2012). https://doi.org/10.1007/s00213-011-2468-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2468-0