Abstract
Background
Several fine-tuned and interconnected hypothalamic peptidergic systems orchestrate the regulation of energy homeostasis in the body. The orexigenic peptide ghrelin and the anorexigenic peptide leptin are among the most important, and both have been implicated in the development of eating disorders from obesity to anorexia nervosa.
Objectives
The goal of these studies was to examine the response of leptin-deficient ob/ob mice in ghrelin-receptor ligands in a food intake task.
Methods
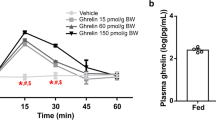
Changes in cumulative food intake were measured after peripheral administration of ghrelin (1 and 2 nmol/10 g) and the ghrelin-receptor antagonist (D-Lys3)-GHRP-6 (66.6 and 133.3 nmol/10 g) in obese and lean control mice during the light and dark cycle as well as in a state of food restriction. Hypothalamic ghrelin and ghrelin-receptor expression was measured in ob/ob and lean mice at two different timepoints.
Results
Ghrelin increased food intake in lean and obese mice in the light and dark cycle, whereas the ghrelin-receptor antagonist caused significantly stronger reduction in food intake in obese mice only in the dark cycle. After fasting, ob/ob mice displayed decreased light cycle sensitivity to the anorexigenic effects of the ghrelin-receptor antagonist. Hypothalamic expression levels of ghrelin were unaltered during the light cycle but decreased during the dark cycle in ob/ob mice; whereas, although unchanged in the light cycle, ghrelin-receptor expression was increased in the dark cycle in obese mice.
Conclusion
The functionality and sensitivity of the ghrelinergic system is dependent on the time of day and the satiety state in leptin-deficient ob/ob mice.





Similar content being viewed by others
References
Archer ZA, Mercer JG (2007) Brain responses to obesogenic diets and diet-induced obesity. Proc Nutr Soc 66:124–130
Ariyasu H, Takaya K, Hosoda H, Iwakura H, Ebihara K, Mori K et al (2002) Delayed short-term secretory regulation of ghrelin in obese animals: evidenced by a specific RIA for the active form of ghrelin. Endocrinology 143:3341–3350
Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T et al (2001) Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86:4753–4758
Arvat E, Maccario M, Di Vito L, Broglio F, Benso A, Gottero C et al (2001) Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab 86:1169–1174
Asakawa A, Inui A, Kaga T, Katsuura G, Fujimiya M, Fujino MA, Kasuga M (2003) Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 52:947–952
Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N et al (2001) Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 120:337–345
Bagnasco M, Kalra PS, Kalra SP (2002) Ghrelin and leptin pulse discharge in fed and fasted rats. Endocrinology 143:726–729
Beck B, Richy S, Stricker-Krongrad A (2004) Feeding response to ghrelin agonist and antagonist in lean and obese Zucker rats. Life Sci 76:473–478
Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal FJ, Krueger JM (2004) Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol 287:R1071–R1079
Brown LM, Benoit SC, Woods SC, Clegg DJ (2007) Intraventricular (i3vt) ghrelin increases food intake in fatty Zucker rats. Peptides 28:612–616
Castañeda TR, Tong J, Datta R, Culler M, Tschöp MH (2010) Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol 31:44–60
Field BC, Wren AM, Cooke D, Bloom SR (2008) Gut hormones as potential new targets for appetite regulation and the treatment of obesity. Drugs 68:147–163
Finger BC, Dinan TG, Cryan JF (2010a) Leptin-deficient mice retain normal appetitive spatial learning yet exhibit marked increases in anxiety-related behaviours. Psychopharmacol Berl 4:559–568
Finger BC, Dinan TG, Cryan JF (2010b) Progressive ratio responding in an obese mouse model: effects of fenfluramine. Neuropharmacology 59:619–626
Horvath TL, Castañeda T, Tang-Christensen M, Pagotto U, Tschöp MH (2003) Ghrelin as a potential anti-obesity target. Curr Pharm Des 9:1383–1395
Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI et al (1996) A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273:974–977
Kitazawa T, De Smet B, Verbeke K, Depoortere I, Peeters TL (2005) Gastric motor effects of peptide and non-peptide ghrelin agonists in mice in vivo and in vitro. Gut 54:1078–1084
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 54:402–408
Luque RM, Huang ZH, Shah B, Mazzone T, Kineman RD (2007) Effects of leptin replacement on hypothalamic-pituitary growth hormone axis function and circulating ghrelin levels in ob/ob mice. Am J Physiol Endocrinol Metab 292:E891–E899
Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S (2001) A role for ghrelin in the central regulation of feeding. Nature 409:194–198
Nogueiras R, Tovar S, Mitchell SE, Rayner DV, Archer ZA, Dieguez C, Williams LM (2004) Regulation of growth hormone secretagogue receptor gene expression in the arcuate nuclei of the rat by leptin and ghrelin. Diabetes 53:2552–2558
Nogueiras R, Tschöp MH, Zigman JM (2008) Central nervous system regulation of energy metabolism: ghrelin versus leptin. Ann NY Acad Sci 1126:14–19
Nonogaki K, Nozue K, Oka Y (2006) Hyperphagia alters expression of hypothalamic 5-HT2C and 5-HT1B receptor genes and plasma des-acyl ghrelin levels in Ay mice. Endocrinology 147:5893–5900
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F (1995) Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543
Pulman KJ, Fry WM, Cottrell GT, Ferguson AV (2006) The subfornical organ: a central target for circulating feeding signals. J Neurosci 26:2022–2030
Rodgers RJ, Holch P, Tallett AJ (2010) Behavioural satiety sequence (BSS): Separating wheat from chaff in the behavioural pharmacology of appetite. Pharmacol Biochem Behav 19:3–14
Schellekens H, Dinan TG, Cryan JF (2010) Lean mean fat reducing “ghrelin” machine: hypothalamic ghrelin and ghrelin receptors as therapeutic targets in obesity. Review. Neuropharmacology 58:2–16
Schiöth HB, Muceniece R, Wikberg JE (1997) Characterization of the binding of MSH-B, HB-228, GHRP-6 and 153N-6 to the human melanocortin receptor subtypes. Neuropeptides 31:565–571
Sun Y, Asnicar M, Saha PK, Chan L, Smith RG (2006) Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metabol 3:379–386
Tschöp M, Smiley DL, Heiman ML (2000) Ghrelin induces adiposity in rodents. Nature 407:908–913
Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA et al (2001) Ghrelin causes hyperphagia and obesity in rats. Diabetes 50:2540–2547
Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J (2004) Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci USA 101:10434–10439
Zheng H, Lenard NR, Shin AC, Berthoud HR (2009) Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 33:S8–S13
Acknowledgements
The authors would like to thank Caroline Brown and Kieran Davey for their excellent technical assistance. The work described herein was supported by Enterprise Ireland under Grant Number CC20080001. The Alimentary Pharmabiotic Centre is a research centre funded by Science Foundation Ireland (SFI), through the Irish Government's National Development Plan. The authors and their work were supported by SFI (grant no.s 02/CE/B124 and 07/CE/B1368). The centre is also funded by GlaxoSmithKline. JFC is funded by European Community's Seventh Framework Programme; Grant Number: FP7/2007-2013, Grant Agreement 201714.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Finger, B.C., Schellekens, H., Dinan, T.G. et al. Is there altered sensitivity to ghrelin-receptor ligands in leptin-deficient mice?: importance of satiety state and time of day. Psychopharmacology 216, 421–429 (2011). https://doi.org/10.1007/s00213-011-2234-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2234-3