Abstract
Rationale
(−)Nicotine produces antinociceptive effects in rodents. meta-Chlorophenylguanidine (MD-354), an analgesia-enhancing agent, binds at 5-HT3 and α2-adrenoceptors and potentiates the antinociceptive effects of an “inactive” dose of clonidine. The present study examined the actions of MD-354 on (−)nicotine-induced antinociception.
Materials and methods
Mouse tail-flick and other assays were employed.
Results
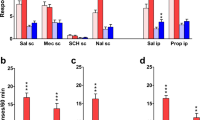
In the tail-flick assay, (−)nicotine (ED50 = 1.66 mg/kg) but not MD-354 produced dose-related antinociceptive effects. Administered in combination with (−)nicotine (2.5 mg/kg), MD-354 (AD50 = 3.4 mg/kg) did not potentiate, but effectively antagonized the antinociceptive actions of (−)nicotine. In a mouse hot-plate assay, MD-354 failed to modify (−)nicotine responses. In combination with a locomotor activity-suppressing dose of (−)nicotine, MD-354 (up to 17 mg/kg) failed to antagonize (−)nicotine-induced hypolocomotion. In a rat drug discrimination paradigm using (−)nicotine as training drug, MD-354 produced saline-appropriate responding; in combination with the training dose of (−)nicotine, MD-354 failed to antagonize the nicotine cue.
Conclusions
MD-354 selectively antagonizes the antinociceptive actions of (−)nicotine in the tail-flick, but not in the hot-plate assay, or either the motor effects, or discriminative stimulus effects of (−)nicotine. The most parsimonious explanation is that MD-354 might act as a negative allosteric modulator of α7 nACh receptors, and radioligand binding and functional data are provided to support this conclusion.






Similar content being viewed by others
References
Abdrakhmanova GR, Ivy Carroll F, Damaj MI, Martin BR (2008) 3′-Fluoro substitution in the pyridine ring of epibatidine improves selectivity and efficacy for α4β2 versus α3β4 nAChRs. Neuropharmacology 55:1287–1292
Arias HR (2000) Localization of agonist and competitive antagonists binding sites on nicotinic acetylcholine receptors. Neurochem Int 36:595–645
Atwell L, Jacobson AE (1978) The search for less harmful analgesics. Lab Anim 7:42–47
Brioni JD, Kim DJ, O’Neill AB (1996) Nicotine cue: lack of effect of the alpha 7 nicotinic receptor antagonist methyllycaconitine. Eur J Pharmacol 301:1–5
Carroll FI, Ma W, Navarro HA, Abraham P, Wolckenhauer SA, Damaj MI, Martin BR (2007) Synthesis, nicotinic acetylcholine receptor binding, antinociceptive and seizure properties of methyllycaconitine analogs. Bioorg Med Chem 15:678–685
Cordero-Erausquin M, Changeux J-P (2001) Tonic modulation of serotonergic transmission in the spinal cord. PANS 98:2803–2807
D’Amour FE, Smith DL (1941) A method for determining loss of pain sensation. J Pharmacol Exp Ther 72:74–79
Damaj MI, Martin BR (1993) Is the dopaminergic system involved in the central effects of nicotine in mice? Psychopharmacology 111:106–108
Damaj MI, Glennon RA, Martin BR (1994) Involvement of the serotonergic system in the hypoactive and antinociceptive effects of nicotine in mice. Brain Res Bull 33:199–203
Damaj MI, Fei-Yin M, Dukat M, Glassco W, Glennon RA, Martin BR (1998) Antinociceptive responses to nicotinic acetylcholine receptor ligands after systemic and intrathecal administration in mice. J Pharmacol Exp Ther 284:1058–1065
Damaj MI, Meyer EM, Martin BR (2000) The antinociceptive effects of α7 nicotinic agonists in an acute pain model. Neuropharmacology 39:2785–2791
Dewey WL, Harris LS, Howes JS, Nuite JA (1970) The effect of various neurohormonal modulations on the activity of morphine and the narcotic antagonist in tail-flick and phenylquinone test. J Pharmacol Exp Ther 175:435–442
Dukat M (2004) 5-HT3 serotonin receptor agonists: a pharmacophoric journey. Curr Med Chem Cent Nerv Syst Agents 4:77–94
Dukat M, Wesolowska A (2005) Antinociception: mechanistic studies on the action of MD-354 and clonidine. Part 1. The 5-HT3 component. Eur J Pharmacol 528:59–64
Dukat M, Abdel-Rahman AA, Ismail AM, Ingher S, Teitler M, Gyermek L, Glennon RA (1996) Structure–activity relationship for the binding of arylpiperazine and arylbiguanides at 5-HT3 serotonin receptors. J Med Chem 39:4017–4026
Dukat M, Young R, Darmani NN, Ahmed B, Glennon RA (2000) The 5-HT3 agent N-3(chlorophenyl)guanidine (MD-354) serves as a discriminative stimulus in rats and displays partial agonist character in a shrew emesis assay. Psychopharmacology 150:200–207
Dukat M, Wesolowska A, Young S, Bondareva T, Young R, Glennon RA (2006) CPDD 2006 Annual Meeting, Scottsdale, Arizona. Abstract 202:51
Dukat M, Glennon RA, Young S (2007) MD-354: what is it good for? CNS Drug Rev 13:1–20
Eddy NB, Leimbach D (1953) Synthetic analgesics. II. Dithienylbutenyl and dithienylbutylamines. J Pharmacol Exp Ther 107:385–393
Finney D (1952) Probit analysis. Cambridge University Press, London
Fozard JR (1994) Role of 5-HT3 receptors in nociception. In: King FD, Jones BJ, Sanger GJ (eds) 5-Hydroxytryptamine-3 receptor antagonists. CRC, Boca Raton, pp 241–253
Freeman GB, Sherman KA, Gibson GE (1987) Locomotor activity as a predictor of times and dosages for studies of nicotine’s neurochemical actions. Pharmacol Biochem Behav 26:305–312
Giordano J, Schultea T (2004) Serotonin 5-HT3 receptor mediation of pain and anti-nociception: implications for clinical therapeutics. Pain Physician 7:141–147
Gommans J, Stolerman IP, Shaib M (2000) Antagonism of the discriminative and aversive stimulus properties of nicotine in C57BL/6J mice. Neuropharmacology 39:2840–2847
Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, Higgins GA (2000) Evidence that nicotinic α7 receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther 294:1112–1119
Gurley DA, Lanthorn TH (1998) Nicotine agonists competitively antagonize serotonin at mouse 5-HT3 receptor expressed in Xenopus oocytes. Neurosci Lett 247:107–110
Hama AT, Lloyd GK, Menzaghi F (2001) The antinociceptive effect of intrathecal administration of epibatidine with clonidine or neostigmine in the formalin test in rats. Pain 91:131–138
Han KJ, Choi SS, Lee JY, Shim EJ, Kwon MS, Seo YJ, Suh HW (2005) Antinociceptive effect of nicotine in various pain models in mouse. Arch Pharm Res 28:209–215
Hao S, Takahata O, Iwasaki H (2001) Antinociceptive interaction between spinal clonidine and lidocaine in the rat formalin test: an isobolographic analysis. Anesth Analg 92:733–738
Hendry JS, Rosecarns JA (1982) Effects of nicotine on conditioned and unconditioned behaviors in experimental animals. Pharmacol Ther 17:431–454
Hirschhorn ID, Rosecrans JA (1974) Studies on the time course and the effect of cholinergic and adrenergic receptor blockers on the stimulus effect of nicotine. Psychopharmacolgia (Berlin) 40:109–120
Iwamoto ET, Marion L (1993) Adrenergic, serotonergic and cholinergic components of nicotinic antinociception in rats. J Pharmacol Exp Ther 265:777–789
Khan IM, Stanislaus S, Zhang L, Taylor P, Yaksh TL (2001) A-85380 and epibatidine each interact with disparate spinal nicotinic receptor subtypes to achieve analgesia and nociception. J Pharmacol Exp Ther 297:230–239
Lichtman AH (1998) The up- and down-method substantially reduces the number of animals required to determine antinociceptive ED50 values. J Pharmacol Tox Meth 40:81–85
Machu TK, Hamilton ME, Frye TF, Shanklin CL, Harris MC, Sun H, Tenner TE Jr, Soti FS, Kem WR (2001) Benzylidene analogs of anabaseine display partial agonist and antagonist properties at the mouse 5-hydroxytryptamine3A receptors. J Pharmacol Exp Ther 299:1112–1119
Mansbach RS, Rovetti CC, Freedland CS (1998) The role of monoamine neurotransmitter systems in the nicotine discriminative stimulus. Drug Alcohol Depend 52:125–134
Marubio LM, Arroyo-Jimenez MM, Cordero-Erausquin M, Lena C, Le Novere N, Huchet M, Damaj MI, Changeux JP (1999) Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature 398:805–810
Maze M, Fujinaga M (2000) α2-Adrenoceptors in pain modulation. Which subtype should be targeted to produce analgesia? Anesthesiology 92:934–936
Moaddel R, Oliveira RV, Kimura T, Hyppolite P, Juhaszova M, Xiao Y, Kellar KJ, Bernier M, Wainer IW (2008) Initial synthesis and characterization of an alpha7 nicotinic receptor cellular membrane affinity chromatography column: effect of receptor subtype and cell type. Anal Chem 80:48–54
Navarro HA, Zhong D, Abraham P, Xu H, Carroll FI (2000) Synthesis and pharmacological characterization of [(125)I]iodomethyllycaconitine ([(125)I]iodo-MLA). A new ligand for the alpha(7) nicotinic acetylcholine receptor. J Med Chem 43:142–145
Navarro HA, Xu H, Zhong D, Abraham P, Carroll FI (2002) In vitro and in vivo characterization of [125I]iodomethyllycaconitine in the rat. Synapse 44:117–123
Rueter LE, Meyer MD, Decker MW (2000) Spinal mechanism underlying A-85380-induced effects on acute thermal pain. Brain Res 872:93–101
Schechter MD, Meehan SM (1992) Further evidence for the mechanisms that may mediate nicotine discrimination. Pharmacol Biochem Behav 41:807–812
Stolerman IP, Garcha HS (1993) Failure of 5-HT3 antagonists and other drugs to block the nicotine discriminative stimulus. NIDA Res Monogr 141:167
Tripathi HL, Martin BR, Aceto MD (1982) Nicotine-induced antinociception in rats and mice: correlation with nicotine brain levels. J Pharmacol Exp Ther 221:91–96
Tritto T, McCallum SE, Waddle SA, Hutton SR, Paylor R, Collins AC, Marks MJ (2004) Null mutant analysis of responses to nicotine: deletion of beta2 nicotinic acetylcholine receptor subunit but not alpha7 subunit reduces sensitivity to nicotine-induced locomotor depression and hypothermia. Nicotine Tob Res 6:145–158
Wesolowska A, Young S, Dukat M (2004) MD-354 potentiates the antinociceptive effect of clonidine in the mouse tail-flick but not hot-plate assay. Eur J Pharmacol 495:129–136
Young R, Glennon RA (1986) Discriminative stimulus properties of amphetamine and structurally related phenalkylamines. Med Res Rev 6:99–130
Young R, Glennon RA (2002) Nicotine and bupropion share a similar discriminative stimulus effect. Eur J Pharmacol 443:113–118
Young S, Vanino N, Sheinin M, Dukat M (2010) Antinociceptive synergism of MD-354 and clonidine. Part II. The α2-adrenoceptor component. Basic Clin Pharmacol Toxicol. doi:10.1111/j.1742-7843.2010.00563.x (Published on line March 28,2010)
Xiao Y, Abdrakhmanova GR, Baydyuk M, Hernandez S, Kellar KJ (2009) Rat neuronal nicotinic acetylcholine receptors containing alpha7 subunit: pharmacological properties of ligand binding and function. Acta Pharmacol Sin 30:842–850
Acknowledgments
We wish to acknowledge T. Bondareva for her assistance with the drug discrimination assay. HEK 293 cells stably expressing rat α7 nAChRs were generously provided by Dr. K. Kellar from Georgetown University.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported in part by J-778 (MD) from the Jeffress Foundation.
Rights and permissions
About this article
Cite this article
Dukat, M., Wesołowska, A., Alley, G. et al. MD-354 selectively antagonizes the antinociceptive effects of (−)nicotine in the mouse tail-flick assay. Psychopharmacology 210, 547–557 (2010). https://doi.org/10.1007/s00213-010-1857-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-1857-0