Abstract
Rationale and objectives
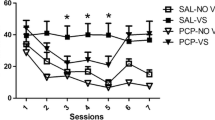
The reinforcement-enhancing effect (REE) of nicotine refers to the drug’s ability to enhance the strength of other primary and conditioned reinforcers. The main aim was to investigate neuropharmacological mechanisms underlying nicotine’s strengthening of a primary visual reinforcer (i.e., a light cue), using a subcutaneous (SC) dose previously shown to provide plasma nicotine levels associated with habitual smoking.
Methods
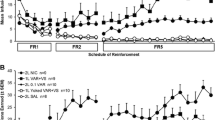
Adult male rats pressed an “active” lever to illuminate a brief cue light during daily 60-min sessions. Rats that showed a clear REE were tested with systemically administered pretreatment drugs followed by nicotine (0.1 mg/kg SC) or saline challenge, in within-subject counterbalanced designs. Pretreatments were mecamylamine (nicotinic, 0.1-1 mg/kg SC), SCH 39166 (D1-like dopaminergic, 0.003-0.2 mg/kg SC), naloxone (opioid, 1 and 5 mg/kg SC), prazosin (alpha1-adrenergic antagonist, 1 and 2 mg/kg IP), rimonabant (CB1 cannabinoid inverse agonist, 3 mg/kg IP), sulpiride (D2-like dopaminergic antagonist, 40 mg/kg SC), or propranolol (beta-adrenergic antagonist, 10 mg/kg IP).
Results
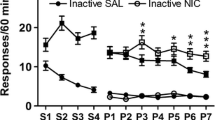
The nicotine REE was abolished by three antagonists at doses that did not impact motor output, i.e., mecamylamine (1 mg/kg), SCH 39166 (0.01 and 0.03 mg/kg), and naloxone (5 mg/kg). Prazosin and rimonabant both attenuated the nicotine REE, but rimonabant also suppressed responding more generally. The nicotine REE was not significantly altered by sulpiride or propranolol.
Conclusions
In adult male rats, the reinforcement-enhancing effect of low-dose nicotine depends on nicotinic receptor stimulation and on neurotransmission via D1/D5 dopaminergic, opioid, alpha1-adrenergic, and CB1 cannabinoid receptors.




Similar content being viewed by others
References
Baker JG (2005) The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 144:317–322
Balle T, Perregaard J, Ramirez MT, Larsen AK, Soby KK, Liljefors T, Andersen K (2003) Synthesis and structure-affinity relationship investigations of 5-heteroaryl-substituted analogues of the antipsychotic sertindole. A new class of highly selective alpha(1) adrenoceptor antagonists. J Med Chem 46:265–283
Barrett ST, Geary TN, Steiner AN, Bevins RA (2016) Sex differences and the role of dopamine receptors in the reward-enhancing effects of nicotine and bupropion. Psychopharmacology 234:187–198
Barrett ST, Geary TN, Steiner AN, Bevins RA (2018) A behavioral economic analysis of the value-enhancing effects of nicotine and varenicline and the role of nicotinic acetylcholine receptors in male and female rats. Behav Pharmacol 29:493–502
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF (2002) Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology 163:230–237
Clarke PBS, Chaudieu I, El-Bizri H, Boksa P, Quik M, Esplin BA, Capek R (1994) The pharmacology of the nicotinic antagonist, chlorisondamine, investigated in rat brain and autonomic ganglion. Br J Pharmacol 111:397–405
Clarke PBS, Kumar R (1984) Effects of nicotine and d-amphetamine on intracranial self-stimulation in a shuttle box test in rats. Psychopharmacology 84:109–114
Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P (2002) SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol 13:451–463
Constantin A, Clarke PBS (2018) Reinforcement enhancement by nicotine in adult rats: behavioral selectivity and relation to mode of delivery and blood nicotine levels. Psychopharmacology 235:641–650
Corrigall WA, Herling S, Coen KM (1988) Evidence for opioid mechanisms in the behavioral effects of nicotine. Psychopharmacology 96:29–35
De Vry J, Schreiber R, Eckel G, Jentzsch KR (2004) Behavioral mechanisms underlying inhibition of food-maintained responding by the cannabinoid receptor antagonist/inverse agonist SR141716A. Eur J Pharmacol 483:55–63
Deroche-Gamonet V, Le MM, Piazza PV, Soubrie P (2001) SR141716, a CB1 receptor antagonist, decreases the sensitivity to the reinforcing effects of electrical brain stimulation in rats. Psychopharmacology 157:254–259
Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF (2003) Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology 169:68–76
Epstein AM, King AC (2004) Naltrexone attenuates acute cigarette smoking behavior. Pharmacol Biochem Behav 77:29–37
Forget B, Coen KM, Le FB (2009) Inhibition of fatty acid amide hydrolase reduces reinstatement of nicotine seeking but not break point for nicotine self-administration--comparison with CB(1) receptor blockade. Psychopharmacology 205:613–624
Forget B, Wertheim C, Mascia P, Pushparaj A, Goldberg SR, Le Foll B (2010) Noradrenergic alpha(1) receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology 35:1751–1760
Fulton HG, Barrett SP (2008) A demonstration of intravenous nicotine self-administration in humans? Neuropsychopharmacology 33:2042–2043
Guy EG, Fisher DC, Higgins GA, Fletcher PJ (2014) Examination of the effects of varenicline, bupropion, lorcaserin, or naltrexone on responding for conditioned reinforcement in nicotine-exposed rats. Behav Pharmacol 25:775–783
Guy EG, Fletcher PJ (2013) Nicotine-induced enhancement of responding for conditioned reinforcement in rats: role of prior nicotine exposure and alpha4beta2 nicotinic receptors. Psychopharmacology 225:429–440
Guy EG, Fletcher PJ (2014) Responding for a conditioned reinforcer, and its enhancement by nicotine, is blocked by dopamine receptor antagonists and a 5-HT receptor agonist but not by a 5-HT receptor antagonist. Pharmacol Biochem Behav 125:40–47
Harris GC, Hedaya MA, Pan WJ, Kalivas P (1996) Beta-adrenergic antagonism alters the behavioral and neurochemical responses to cocaine. Neuropsychopharmacology 14:195–204
Harrison AA, Gasparini F, Markou A (2002) Nicotine potentiation of brain stimulation reward reversed by DHbetaE and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology 160:56–66
Huston-Lyons D, Kornetsky C (1992) Effects of nicotine on the threshold for rewarding brain stimulation in rats. Pharmacol Biochem Behav 41:755–759
Ismayilova N, Shoaib M (2010) Alteration of intravenous nicotine self-administration by opioid receptor agonist and antagonists in rats. Psychopharmacology 210:211–220
Ivanová S, Greenshaw AJ (1997) Nicotine-induced decreases in VTA electrical self-stimulation thresholds: blockade by haloperidol and mecamylamine but not scopolamine or ondansetron. Psychopharmacology 134:187–192
Jensen KP, DeVito E, Valentine G, Gueorguieva R, Sofuoglu M (2016) IV nicotine self-administration in smokers: dose-response function and sex differences. Neuropsychopharmacology 41:2034–2040
Kirshenbaum A, Green J, Fay M, Parks A, Phillips J, Stone J, Roy T (2014) Reinforcer devaluation as a consequence of acute nicotine exposure and withdrawal. Psychopharmacology 232:1583–1594
Kirshenbaum AP, Suhaka JA, Phillips JL, de Souza Pinto MV (2016) Nicotine enhancement and reinforcer devaluation: interaction with opioid receptors. Pharmacol Biochem Behav 150-151:1–7
Levin ME, Weaver MT, Palmatier MI, Caggiula AR, Sved AF, Donny EC (2012) Varenicline dose dependently enhances responding for nonpharmacological reinforcers and attenuates the reinforcement-enhancing effects of nicotine. Nicotine Tob Res 14:299–305
Liu X, Palmatier MI, Caggiula AR, Donny EC, Sved AF (2007) Reinforcement enhancing effect of nicotine and its attenuation by nicotinic antagonists in rats. Psychopharmacology 194:463–473
Morgenstern R, Fink H, Oelssner W (1983) LSD-potentiated apomorphine hypermotility: a model for differentiating antipsychotic drugs. Pharmacol Biochem Behav 18:13–17
Nguyen T, Thomas BF, Zhang Y (2019) Overcoming the psychiatric side effects of the cannabinoid CB1 receptor antagonists: current approaches for therapeutics development. Curr Top Med Chem 19:1418–1435
Ogren SO, Fuxe K (1988) Apomorphine and pergolide induce hypothermia by stimulation of dopamine D-2 receptors. Acta Physiol Scand 133:91–95
Ogren SO, Hall H, Kohler C, Magnusson O, Sjostrand SE (1986) The selective dopamine D2 receptor antagonist raclopride discriminates between dopamine-mediated motor functions. Psychopharmacology 90:287–294
Olausson P, Jentsch JD, Taylor JR (2004) Nicotine enhances responding with conditioned reinforcement. Psychopharmacology 171:173–178
Pak AC, Ashby CR, Heidbreder CA, Pilla M, Gilbert J, Xi ZX, Gardner EL (2006) The selective dopamine D3 receptor antagonist SB-277011A reduces nicotine-enhanced brain reward and nicotine-paired environmental cue functions. Int J Neuropsychopharmacol 9:585–602
Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF (2006) Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology 184:391–400
Palmatier MI, Kellicut MR, Brianna SA, Brown RW, Robinson DL (2014) The incentive amplifying effects of nicotine are reduced by selective and non-selective dopamine antagonists in rats. Pharmacol Biochem Behav 126:50–62
Palmatier MI, Levin ME, Mays KL, Donny EC, Caggiula AR, Sved AF (2009) Bupropion and nicotine enhance responding for nondrug reinforcers via dissociable pharmacological mechanisms in rats. Psychopharmacology 207:381–390
Palmatier MI, Liu X, Donny EC, Caggiula AR, Sved AF (2007) Metabotropic glutamate 5 receptor (mGluR5) antagonists decrease nicotine seeking, but do not affect the reinforcement enhancing effects of nicotine. Neuropsychopharmacology 33:2139–2147
Papke RL, Sanberg PR, Shytle RD (2001) Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J Pharmacol Exp Ther 297:646–656
Paterson NE (2009) The neuropharmacological substrates of nicotine reward: reinforcing versus reinforcement-enhancing effects of nicotine. Behav Pharmacol 20:211–225
Perkins KA, Karelitz JL, Boldry MC (2017) Nicotine acutely enhances reinforcement from non-drug rewards in humans. Front Psychiatry 8: article 65
Rollema H, Hurst RS (2018) The contribution of agonist and antagonist activities of alpha4beta2* nAChR ligands to smoking cessation efficacy: a quantitative analysis of literature data. Psychopharmacology 235:2479–2505
Rose JE (2008) Disrupting nicotine reinforcement: from cigarette to brain. Ann N Y Acad Sci 1141:233–256
Rose JE, Salley A, Behm FM, Bates JE, Westman EC (2010) Reinforcing effects of nicotine and non-nicotine components of cigarette smoke. Psychopharmacology 210:1–12
Rupprecht LE, Smith TT, Schassburger RL, Buffalari DM, Sved AF, Donny EC (2015) Behavioral mechanisms underlying nicotine reinforcement. Curr Top Behav Neurosci 24:19–53
Seeman P, Van Tol HHM (1994) Dopamine receptor pharmacology. Trends Pharmacol Sci 15:264–270
Sorge RE, Clarke PB (2009) Rats self-administer intravenous nicotine delivered in a novel smoking-relevant procedure: effects of dopamine antagonists. J Pharmacol Exp Ther 330:633–640
Sorge RE, Pierre VJ, Clarke PB (2009) Facilitation of intravenous nicotine self-administration in rats by a motivationally neutral sensory stimulus. Psychopharmacology 207:191–200
Spiller K, Xi ZX, Li X, Ashby CR Jr, Callahan PM, Tehim A, Gardner EL (2009) Varenicline attenuates nicotine-enhanced brain-stimulation reward by activation of alpha4beta2 nicotinic receptors in rats. Neuropharmacology 57:60–66
Tobey KM, Walentiny DM, Wiley JL, Carroll FI, Damaj MI, Azar MR, Koob GF, George O, Harris LS, Vann RE (2012) Effects of the specific α4β2 nAChR antagonist, 2-fluoro-3-(4-nitrophenyl) deschloroepibatidine, on nicotine reward-related behaviors in rats and mice. Psychopharmacology 223:159–168
Trovero F, Blanc G, Herve D, Vezina P, Glowinski J, Tassin JP (1992) Contribution of an alpha 1-adrenergic receptor subtype to the expression of the “ventral tegmental area syndrome”. Neuroscience 47:69–76
Wamsley JK, Hunt ME, McQuade RD, Alburges ME (1991) [3H]SCH39166, a D1 dopamine receptor antagonist: binding characteristics and localization. Exp Neurol 111:145–151
Wing VC, Shoaib M (2010) A second-order schedule of food reinforcement in rats to examine the role of CB1 receptors in the reinforcement-enhancing effects of nicotine. Addict Biol 15:380–392
Wright JM, Dobosiewicz MR, Clarke PB (2012) Alpha- and beta-adrenergic receptors differentially modulate the emission of spontaneous and amphetamine induced 50-kHz ultrasonic vocalizations in adult rats. Neuropsychopharmacology 37:808–821
Wright JM, Ren S, Constantin A, Clarke PBS (2018) Enhancement of a visual reinforcer by D-amphetamine and nicotine in adult rats: relation to habituation and food restriction. Psychopharmacology 235:803–814
Acknowledgements
Supported by the Canadian Institutes of Health Research of Canada (operating grant 156045, to P.B.S.C.) and by Undergraduate Student Research Awards to SR and TMVC (the Natural Sciences and Engineering Research Council of Canada and Fonds de la Recherche en Santé du Québec, respectively). P.B.S.C. is a member of the Center for Studies in Behavioral Neurobiology at Concordia University, Montreal. The authors have no financial relationship with the organizations that sponsored this research. All experiments comply with the current laws of Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All research procedures were reviewed and approved by the McGill Animal Care Committee in accordance with the guidelines of the Canadian Council on Animal Care.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Satanove, D.J., Rahman, S., Chan, T.M.V. et al. Nicotine-induced enhancement of a sensory reinforcer in adult rats: antagonist pretreatment effects. Psychopharmacology 238, 475–486 (2021). https://doi.org/10.1007/s00213-020-05696-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05696-5