Abstract
Rationale
The discriminative stimulus properties of clozapine (CLZ) have been studied for decades because it remains the prototype for atypical antipsychotic drug effects and yet is unique in many ways, including increased efficacy in treatment-resistant schizophrenia and in reducing suicidality. Recent studies have indicated that the active CLZ metabolite N-desmethylclozapine (NDMC) may play a role in mediating the cognitive efficacy of CLZ and may also have atypical antipsychotic properties.
Objectives
The present study sought to determine if NDMC has discriminative stimulus properties similar to that of its parent drug CLZ.
Materials and methods
Rats were trained to discriminate 1.25 mg/kg CLZ from vehicle in a two-choice drug discrimination task.
Results
Although NDMC (2.5–20.0 mg/kg) failed to substitute for CLZ, the combination of NDMC (5.0 and 10.0 mg/kg) with a low dose (0.3125 mg/kg) of CLZ produced full substitution (>80% CLZ-appropriate responding) for the 1.25 mg/kg CLZ training dose. Co-administration of the M1-preferring receptor antagonist trihexyphenidyl (6.0 mg/kg) with a 5.0 mg/kg dose of NDMC produced partial substitution (>60% to <80% CLZ-appropriate responding) for CLZ, while administration of trihexyphenidyl alone (0.3–12.0 mg/kg) failed to substitute for CLZ.
Conclusions
These findings suggest that NDMC produces discriminative stimulus effects that are different from those elicited by its parent drug CLZ. This difference may be due to the agonist properties of NDMC at M1 muscarinic cholinergic receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clozapine (CLZ) is classified as an atypical antipsychotic drug based on a low incidence of extrapyramidal motor side effects, effectiveness for treating negative symptoms (as well as positive symptoms), and reduced cognitive deficits associated with schizophrenia (Young et al. 1997; Wahlbeck et al. 2008). Furthermore, the clinical efficacy of CLZ is often considered to be superior to other atypical antipsychotic drugs in several regards, including (1) increased efficacy in treatment-resistant patients (Kane et al. 1988), (2) reduced suicidality (Meltzer et al. 2003), and (3) reduction of psychosis in Parkinson patients treated with l-dopa (without exacerbating motor symptoms; Scholz and Dichgans 1985; Meltzer et al. 1995).
The major active metabolite of CLZ, N-desmethylclozapine (NDMC), also may have atypical antipsychotic properties as evidenced in both preclinical (Li et al. 2005; Lameh et al. 2007) and clinical studies (Tamminga et al. 2006). For example, NDMC reverses hyperactivity induced by the NMDA receptor antagonist MK-801 and by the dopamine (DA) agonist amphetamine (see Lameh et al. 2007) and the first clinical trials in schizophrenic patients with NDMC demonstrated safety and tolerability and signs of antipsychotic activity (Tamminga et al. 2006).
Lameh et al. (2007) have shown that both CLZ and NDMC act as potent antagonists at serotonin (5-HT)2A receptors with a weaker affinity for dopamine D2 receptors. However, there appear to be important differences with regard to NDMC as compared to other atypical antipsychotic drugs. For example, NDMC is a partial agonist at DA D2 receptors, and CLZ has been shown to antagonize NDMC-induced D2 receptor activation (Burstein et al. 2005). An arguably more important difference in receptor pharmacology between CLZ and NDMC is that CLZ is usually reported to be an antagonist (Schotte et al. 1996) or a partial agonist (Michal et al. 1999; Olianas et al. 1999) at muscarinic receptors, while NDMC is a potent partial agonist at muscarinic receptors, with greater intrinsic efficacy at M1, M2, and M4 receptors as compared to CLZ (Weiner et al. 2004). Although CLZ exhibits a high affinity for muscarinic receptors in vitro, other studies have suggested that the in vivo affinity of CLZ for muscarinic receptors may be considerably weaker (Arnt and Skarsfeldt 1998; Bymaster and Falcone 2000); however, this is inconsistent with some of the preclinical effects of clozapine (e.g., Kelley and Porter 1997).
This difference in intrinsic efficacy at muscarinic receptors between CLZ and NDMC may be relevant to the treatment efficacy of CLZ. It is well established that muscarinic antagonism can cause deficits in cognitive function (Riedel et al. 1995; Green et al. 2005; Plakke et al. 2008); yet, CLZ is attributed with an ability to improve cognitive function. Interestingly, recent reports indicate that larger ratios of NDMC/CLZ in plasma predict greater improvements in cognitive function (Weiner et al. 2004). Thus, it may be the case that higher ratios of NDMC/CLZ help to overcome the muscarinic antagonist properties of CLZ and allow for improvement of cognitive deficits.
When studied in the drug discrimination procedure, a behavioral model used to assess receptor-mediated interoceptive effects, CLZ generally appears to produce interoceptive effects indicative of antagonism at muscarinic receptors. However, the ability of muscarinic receptor antagonists to produce CLZ-appropriate responding in this task depends on the training dose of CLZ and the species used. The nonselective muscarinic receptor antagonist atropine has produced partial substitution, and the nonselective muscarinic receptor antagonist scopolamine has produced full substitution to 5.76 and 5.0 mg/kg CLZ (i.p.) discriminative stimuli in rats (Nielsen 1988; Kelley and Porter 1997; Goudie et al. 1998). In addition, scopolamine has produced full substitution for CLZ (98.5%) in rats trained to discriminate 5.0 mg/kg CLZ versus 1.0 mg/kg chlorpromazine versus vehicle (i.p.) in a three-choice drug discrimination task (Porter et al. 2005). However, atropine produced minimal substitution for 6.0 mg/kg CLZ (p.o.) in rats (Goas and Boston 1978). In pigeons, atropine and scopolamine (i.m.) failed to substitute for 1.0 mg/kg CLZ (Hoenicke et al. 1992), and in C57BL/6 mice, scopolamine (s.c.) failed to substitute for 2.5 mg/kg CLZ (Philibin et al. 2005). In rats trained to discriminate 1.25 mg/kg CLZ versus 5.0 mg/kg CLZ versus CLZ vehicle in a three-choice drug discrimination task, partial stimulus generalization to scopolamine (60–75% CLZ-appropriate responding) occurred from the 5.0 mg/kg CLZ discriminative stimulus, while the remaining percentages of responding occurred primarily on the 1.25 mg/kg CLZ-appropriate lever (Prus et al. 2006). No stimulus generalization occurred from either the 1.25 or the 5.0 mg/kg doses of CLZ to NDMC at doses up to 8.0 mg/kg in this study, although it should be noted that NDMC was not tested up to doses that produced rate suppressant effects (Prus et al. 2006). The M1 receptor-preferring antagonist trihexyphenidyl has been reported to substitute for both 5.0 and 1.25 mg/kg CLZ doses in two-choice drug discrimination tasks (Kelley and Porter 1997; Prus et al. 2004, respectively), while the M2 receptor antagonist BIBN 99 does not (5.0 mg/kg CLZ dose; Kelley and Porter 1997).
While species, procedural, and training differences may account for the ability of antipsychotic drugs or selective ligands to substitute for CLZ (or not substitute in some situations), these findings also indicate that muscarinic receptor antagonists are capable of producing substitution to CLZ and that the anti-muscarinic properties of CLZ dramatically influence the interoceptive cue in some contexts. The present study sought to determine if the active CLZ metabolite and putative atypical antipsychotic drug NDMC had discriminative stimulus properties similar to that of its parent drug CLZ and also examined the role of M1 antagonism in the discriminative stimulus properties of a low training dose (1.25 mg/kg) of CLZ and NDMC. A low training dose of CLZ was chosen because muscarinic receptor antagonism appears to be less important for the low-dose discriminative stimulus effects of CLZ as compared to a higher training dose of CLZ (e.g., 5.0 mg/kg) in rats (Porter, unpublished data). Given the fact that NDMC is a partial agonist at muscarinic receptors rather than an antagonist, we felt that the 1.25 mg/kg training dose conferred a greater chance for NDMC to substitute for the CLZ cue. Finally, low training doses of CLZ produce generalization to more atypical antipsychotic drugs than do higher training doses (Porter et al. 2000; Prus et al. 2005), thus making this a better model for screening putative atypical antipsychotic drugs.
Materials and methods
Subjects
Eight male Sprague–Dawley rats (Harlan Breeding Laboratories, Indianapolis, IN, USA; 300–350 g) were individually housed in constant room temperature conditions (22–24°C) under a 12-h light/dark cycle (0600–1800 hours). Rats were maintained at 85% of free-feeding weight through restricted food rations, but access to water was available ad libitum. The “Guide for the Care and Use of Laboratory Animals” (National Research Council 2003) was followed, and all procedures were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
Apparatus
Standard two-lever (retractable) rat operant conditioning chambers contained in sound-attenuating cabinets equipped with fans for ventilation and masking noise (Model ENV-008-VP; MED Associates, St Albans, VT, USA) were used for all drug discrimination sessions. Reinforcers consisted of 45-mg food pellets (Dustless Precision Pellets, F0021, Bio-Serv, Frenchtown, NJ, USA).
Drugs
CLZ (gift from Novartis, Hanover, NJ, USA), was dissolved in deionized water with one to two drops of lactic acid. Both trihexyphenidyl (Sigma-Aldrich, St. Louis, MO, USA) and NDMC (Sigma-Aldrich) were dissolved in deionized water only. CLZ was administered 1 h prior to test sessions, and trihexyphenidyl and NDMC were administered 30 min prior to test sessions. Vehicle control injections were administered at the appropriate pretreatment times for each drug. All doses were injected intraperitoneally at a volume of 1 ml/kg. Injection routes, presession injection times, and doses for these drugs were based on previous studies from this laboratory.
Drug discrimination training
After lever press training, rats were trained in “errorless” sessions where only the condition-appropriate lever was present for five consecutive days, first with vehicle and then for 5 days with CLZ using the fixed ratio (FR) 30 reinforcement schedule (see Porter et al. 2000; Prus et al. 2005 for additional training details). These and all other sessions in the study were 15 min in length. After this, two-lever training sessions were conducted in order to train the rats to discriminate 1.25 mg/kg clozapine from vehicle. During two-lever training sessions, emitting 30 consecutive responses on the condition-appropriate lever resulted in pellet delivery, while responses on the other lever reset the FR response counter on the condition-appropriate lever but had no other programmed consequences. The training criteria consisted of passing five of six consecutive sessions of (1) completing the first 30 consecutive responses on the condition-appropriate lever, (2) making 80% or greater of all responses on the condition-appropriate lever, and (3) maintaining a response rate of at least 30 responses per minute (RPM).
Drug testing
Before each test session, rats had to meet all three training criteria during the two training sessions that immediately proceeded the test day. A test session was similar to a training session, except that responding on both levers was reinforced according to the FR 30 reinforcement schedule (responses on either lever still reset the FR response requirement on the other lever). The drugs were tested in the following order: CLZ (N = 8), NDMC (N = 8), 0.3125 mg/kg CLZ NDMC (N = 7), trihexyphenidyl (N = 5), and 5.0 mg/kg NDMC trihexyphenidyl (N = 4). Control tests (with a minimum of two training days before each test) with the 1.25 mg/kg CLZ training dose and vehicle were conducted prior to each drug in order to determine that the CLZ discrimination was still being maintained by the rats. Any rats that did not maintain good stimulus control (as determined by the control tests) were removed from the study. The doses for each drug were tested in ascending order from low to high until either full substitution for CLZ was evident (see “Data analysis” below) or there was a significant decrease in response rate.
Data analysis
he lever on which the first FR 30 was completed, percent lever responding for each drug condition, and the RPM were recorded for every training and test session. Percent condition-appropriate responding and RPM were reported as means [±the standard error of the mean (SEM)] in dose–effect curves. Full substitution was defined as 80% or greater condition-appropriate responding, and partial substitution was defined as greater than or equal to 60% and less than 80% condition-appropriate responding (Prus et al. 2005). For drugs that produced full substitution for 1.25 mg/kg CLZ, ED50 values with 95% confidence intervals (CI) were obtained for dose–response curves using a least squares linear regression analysis. If an animal’s response rate fell below five RPM, its percent lever responding data were excluded from the dose–response curve and the ED50 calculations. A one-factor repeated measures analysis of variance (ANOVA) was conducted to assess rate suppressant effects, and for significant F values, Newman–Keuls multiple comparison tests were conducted to identify rate-suppressant doses relative to vehicle (GB Stat software, Version 10.0, 2004; Dynamic Microsystems, Silver Spring, MD, USA).
Results
Clozapine
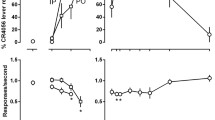
The 1.25 mg/kg training dose of CLZ fully generalized to both 1.25 and 5.0 mg/kg CLZ (ED50 = 0.43 mg/kg, 95% CI = 0.27–0.68 mg/kg) producing nearly 100% drug-lever responding at both doses (Fig. 1). A small, but statistically significant decrease in RPM was found at the 5.0-mg/kg dose of CLZ as compared to vehicle, F 6,42 = 5.02, p < 0.001.
The atypical antipsychotic drug clozapine (CLZ; 0.078 mg/kg–5.0 mg/kg, i.p.) was tested for substitution in rats trained to discriminate 1.25 mg/kg CLZ from vehicle (VEH) in a two-lever drug discrimination task. Mean percent drug lever responding (+SEM) (filled symbols) are shown on the left ordinate and the mean responses per minute (+SEM) (empty symbols) are shown on the right ordinate for the vehicle (VEH) and 1.25 mg/kg CLZ control points and for each dose of CLZ tested. Rats that failed to emit a response rate of at least five responses per minute were excluded from the mean percent drug lever calculation; otherwise, the number of subjects represented at each point is equal to N. For the response rate data, statistically significant differences compared to VEH are indicated by asterisks (*p < 0.05, **p < 0.01)
N-desmethylclozapine
NDMC (1.25–20.0 mg/kg) failed to substitute for CLZ up to a dose (20.0 mg/kg) that produced a significant reduction in response rates, F 6,24 = 9.32, p < 0.001 (Fig. 2).
Substitution testing for the active CLZ metabolite and putative atypical antipsychotic drug N-desmethylclozapine (NDMC; 1.25–20.0 mg/kg, i.p.). See Fig. 1 for further details
N-Desmethylclozapine + 0.3125 mg/kg clozapine
In order to determine if NDMC could potentiate the discriminative stimulus effects of CLZ, the 0.3125-mg/kg dose of CLZ (which produced only 33% CLZ-appropriate responding when CLZ was tested for generalization) was combined with NDMC (2.5–10.0 mg/kg). Combinations of 5.0 and 10.0 mg/kg NDMC with 0.3125 mg/kg CLZ produced full substitution (ED50 = 1.68 mg/kg, 95% CI = 0.94–2.99 mg/kg) for the CLZ discriminative stimulus (Fig. 3). The 10.0 mg/kg NDMC in combination with 0.3125 mg/kg CLZ produced a significant decrease in response rates, F 5,30 = 4.42, p < 0.01.
Substitution testing for combined administration of NDMC (2.5–10.0 mg/kg, i.p) with CLZ (0.3125 mg/kg, i.p.). See Fig. 1 for further details
Trihexyphenidyl
Trihexyphenidyl (0.3–12.0 mg/kg) failed to substitute for the 1.25 mg/kg training dose of CLZ (Fig. 4). The 12.0-mg/kg dose of trihexyphenidyl produced a significant reduction in response rates (F 6,24 = 3.52, p < 0.05), which prevented the testing of higher doses of this compound.
Substitution testing for the M1-preferring muscarinic receptor antagonist trihexyphenidyl (0.3–12.0 mg/kg, i.p.). See Fig. 1 for further details
Trihexyphenidyl + 5.0 mg/kg N-desmethylclozapine
In order to determine if blockade of the muscarinic M1 receptor agonist effects of NDMC could potentiate the amount of stimulus generalization from the CLZ discriminative stimulus, the 5.0 mg/kg dose of NDMC (which produced only 37.5% CLZ-appropriate responding when tested alone, see Fig. 2) was combined with trihexyphenidyl (1.0–12.0 mg/kg). The combination of 6.0 mg/kg trihexyphenidyl with 5.0 mg/kg NDMC produced partial substitution (72.1% CLZ-appropriate responding) for CLZ (Fig. 5). A significant decrease in response rates was observed with the 6.0 mg/kg trihexyphenidyl plus 5.0 mg/kg NDMC and the 12.0 mg/kg trihexyphenidyl plus 5.0 mg/kg NDMC dose combinations, F 5, 20 = 21.76, p < 0.001. The 12.0 mg/kg trihexyphenidyl plus 5.0 mg/kg NDMC combination completely suppressed response rates; therefore, these data were not included in the dose–response curve for percent lever selection and no response rate data are shown in Fig 2.
Substitution testing for combined administration of NDMC (5.0 mg/kg, i.p.) with trihexyphenidyl (1.0–6.0 mg/kg, i.p.). See Fig. 1 for further details
Discussion
The present study sought to determine if the active CLZ metabolite and putative atypical antipsychotic drug NDMC has discriminative stimulus properties similar to that of its parent drug CLZ. NDMC failed to produce CLZ-appropriate responding for the 1.25 mg/kg CLZ discriminative stimulus up to a dose (20.0 mg/kg) that produced rate-disruptive effects, indicating that NMDC itself does not mediate the interoceptive effects of CLZ. These findings are consistent with a previous study using rats trained to discriminate 1.25 mg/kg CLZ versus 5.0 mg/kg CLZ versus vehicle in a three-choice drug discrimination task (Prus et al. 2006), although this earlier study did not test doses of NDMC above 8.0 mg/kg. The receptor pharmacology of CLZ and NDMC are generally similar, except at muscarinic receptors, where CLZ is an antagonist or weak partial agonist at muscarinic receptors, while NDMC is a potent partial agonist at muscarinic receptors (Weiner et al. 2004; Li et al. 2005; Lameh et al. 2007). Thus, differences in the actions of CLZ and NDMC at muscarinic receptor binding may have accounted for the inability of NDMC to substitute for 1.25 mg/kg CLZ in the present and previous (Prus et al. 2006) studies.
Indeed, the muscarinic M1-preferring receptor antagonist trihexyphenidyl failed to substitute for the 1.25-mg/kg training dose of CLZ in the present study. While higher training doses of CLZ appear to elicit discriminative stimulus effects through muscarinic receptor antagonism, the discriminative stimulus effects of the lower training dose used in the present study appears to be less dependent on muscarinic receptor antagonism. Full substitution for a higher CLZ training dose (5.0 mg/kg) in rats has been found with the muscarinic receptor antagonists scopolamine (Goudie et al. 1998; Kelley and Porter 1997) and trihexyphenidyl (Kelley and Porter 1997), and partial substitution for a similar training dose of CLZ has been reported using atropine (Nielsen 1988). However, trihexyphenidyl was found to produce full substitution for 1.25 mg/kg CLZ in a two-lever drug discrimination (Prus et al. 2004), and in a three-choice drug discrimination study that trained rats to discriminate 1.25 mg/kg CLZ versus 5.0 mg/kg CLZ versus vehicle, scopolamine produced partial substitution for the 5.0 mg/kg CLZ training dose, while the remaining percentage of responses occurred mainly on the 1.25 mg/kg CLZ-appropriate lever (Prus et al. 2006). Thus, while stimulus properties mediated by muscarinic receptor antagonism appear to be more prominent in the 5.0 mg/kg CLZ discriminative stimulus, they may be present to a lesser degree in the 1.25 mg/kg CLZ discriminative stimulus.
Combined administration of trihexyphenidyl with NDMC in the present study resulted in partial substitution for CLZ (72.1% CLZ-appropriate responding), suggesting that the muscarinic receptor partial agonist effects of NDMC may have been attenuated (at least partially) by the muscarinic receptor antagonist effects of trihexyphenidyl. Thus, any CLZ-like stimulus effects produced by NDMC may be due to actions at non-muscarinic receptors. This may explain the full substitution for CLZ found when NDMC was combined with a low dose of CLZ (0.3125 mg/kg). However, the role of other receptor mechanisms involved in NDMC fully substituting for CLZ, in combination with a low dose of CLZ, may be difficult to determine since no selective receptor ligands, with the exception of trihexyphenidyl (Prus et al. 2004), have been shown to produce full substitution for the 1.25 mg/kg CLZ training dose in rats (Porter et al. 2000; Prus et al. 2004; Prus et al. 2006). Interestingly, combined administration of a 5-HT2A receptor antagonist with the D2-receptor preferring antagonist and typical antipsychotic drug haloperidol has been shown to produce full substitution for a 1.25-mg/kg CLZ training dose, although this effect occurred at only one-dose combination (Prus et al. 2004). It is clear that CLZ’s discriminative cue is complex and represents a compound cue mediated by actions at two or more receptors (see Goudie et al. 1998; Goudie and Smith 1999; Porter et al. 2000).
An important consideration when comparing the effects of NDMC and CLZ is the route of injection. In the present study, rats were trained to discriminate intraperitoneally administered CLZ, which subjects the drug to first pass metabolism. While NDMC does not appear to mediate the interoceptive effects of CLZ (as NDMC failed to substitute for CLZ), it is possible that NDMC contributed to the interoceptive cue properties of CLZ when using the intraperitoneal injection route. Considering that drug injections occur sometime before the test session, metabolism of CLZ would certainly allow time for NDMC to enter the central nervous system and become behaviorally active.
The current study provided experimental evidence that CLZ and NDMC exhibit differences in interoceptive effects that are likely due, at least in part, to their different actions at muscarinic receptors; however, the combination of NDMC with a low, non-substituting dose of CLZ was sufficient to engender CLZ-appropriate responding. The combination of these two compounds also may be important with regard to clinical effects, based on a study by Weiner et al. (2004), which found that greater NDMC/CLZ ratios in serum predicted improvements in cognitive functioning and quality of life in CLZ-treated patients with schizophrenia. It has been further suggested by Davies et al. (2005) that the M1 receptor partial agonist effects of NDMC may mediate the unique clinical efficacy of clozapine, possibly through overcoming blockade of muscarinic receptors produced by CLZ. Despite these differences, the full substitution for CLZ found when NDMC was combined with a low dose of CLZ, indicates that certain interoceptive effects of NDMC are similar to those produced by CLZ. Thus, NDMC, like its parent drug CLZ (Goudie et al. 1998; Goudie and Smith 1999; Porter et al. 2000), may have a compound discriminative cue. The potential antipsychotic efficacy of NDMC has led to testing of NDMC for possible clinical use in the USA, under the name ACP-104. In the only released study to date, ACP-104 was reported to produce improvements on the total score and positive symptom subscale on the Positive and Negative Syndrome Scale in a preliminary tolerability and efficacy study in schizophrenic patients (Tamminga et al. 2006).
The present study represents the first co-administration of CLZ and its active metabolite NDMC in the context of a drug discrimination task and helps to lay the foundation for a more thorough understanding of the discriminative stimulus properties of antipsychotic drugs and their active metabolites. For example, if animals can be trained to discriminate NDMC from vehicle, then cross-substitution studies could provide important information comparing the discriminative stimulus properties of CLZ and NDMC. Such future studies would be useful for identifying the receptor mechanisms that may account for the similarities and differences between the behavioral effects of CLZ and NDMC.
References
Arnt J, Skarsfeldt T (1998) Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology 18:63–101
Burstein ES, Ma J, Wong S, Gao Y, Pham E, Knapp AE, Nash NR, Olsson R, Davis RE, Hacksell U, Weiner DM, Brann MR (2005) Intrinsic efficacy of antipsychotics at human D2, D3, and D4 dopamine receptors: identification of the clozapine metabolite N-desmethylclozapine as a D2/D3 partial agonist. J of Pharmacol Exp Ther 315:1278–1287
Bymaster FP, Falcone JF (2000) Decreased binding affinity of olanzapine and clozapine for human muscarinic receptors in intact clonal cells in physiological medium. Eur J Pharmacology 390:245–248
Davies MA, Compton-Toth BA, Hufeisen SJ, Meltzer HY, Roth BL (2005) The highly efficacious actions of N-desmethylclozapine at muscarinic receptors are unique and not a common property of either typical or atypical antipsychotic drugs: is M1 agonism a pre-requisite for mimicking clozapine’s actions. Psychopharmacology 178:451–460
Goas JA, Boston JE (1978) Discriminative stimulus properties of clozapine and chlorpromazine. Pharmacol Biochem Behav 8:235–241
Goudie AJ, Smith JA (1999) Discriminative stimulus properties of antipsychotics. Pharmacol Biochem Behav 64(2):193–201
Goudie AJ, Smith JA, Taylor A, Taylor MA, Tricklebank MD (1998) Discriminative stimulus properties of the atypical neuroleptic clozapine in rats: tests with subtype selective receptor ligands. Behav Pharmacol 9:699–710
Green A, Ellis KA, Ellis J, Bartholomeusz CF, Ilic S, Croft RJ, Phan KL, Nathan PJ (2005) Muscarinic and nicotinic receptor modulation of object and spatial n-back working memory in humans. Pharmacol Biochem Behav 81(3):575–584
Hoenicke EM, Vanecek SA, Woods JH (1992) The discriminative stimulus effects of clozapine in pigeons: involvement of 5-hydroxytryptamine1C and 5-hydroxytryptamine2 receptors. J of Pharmacol Exp Ther 263:276–284
Kane JM, Honigfeld G, Singer J, Meltzer H (1988) Clozapine in treatment-resistant schizophrenics. Psychopharmacol Bull 24:62–67
Kelley BM, Porter JH (1997) The role of muscarinic cholinergic receptors in the discriminative stimulus properties of clozapine in rats. Pharmacol Biochem Behav 57:707–719
Lameh J, Burstein ES, Taylor E, Weiner DM, Vanover KE, Bonhaus DW (2007) Pharmacology of N-desmethylclozapine. Pharmacol Ther 115:223–231
Li Z, Huang M, Ichikawa J, Dai J, Meltzer HY (2005) N-desmethylclozapine, a major metabolite of clozapine, increases cortical acetylcholine and dopamine release in vivo via stimulation of M1 muscarinic receptors. Neuropsychopharmacology 30:1986–1995
Meltzer HY, Kennedy J, Dai J, Parsa M, Riley D (1995) Plasma clozapine levels and the treatment of L-DOPA-induced psychosis in Parkinson’s disease. A high potency effect of clozapine. Neuropsychopharmacology 12:39–45
Meltzer HY, Alphs L, Green AI, Altamura AC, Anand R, Bertoldi A, Bourgeois M, Chouinard G, Islam MZ, Kane J, Krishnan R, Lindenmayer JP, Potkin S (2003) Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 60:82–91
Michal P, Lysikova M, El-Fakahany EE, Tucek S (1999) Clozapine interaction with the M2 and M4 subtypes of muscarinic receptors. Eur J Pharmacol 376:119–125
Nielsen EB (1988) Cholinergic mediation of the discriminative stimulus properties of clozapine. Psychopharmacology (Berl) 94:115–118
Olianas MC, Maullu C, Onali P (1999) Mixed agonist-antagonist properties of clozapine at different human cloned muscarinic receptor subtypes expressed in Chinese hamster ovary cells. Neuropsychopharmacology 20:263–270
Philibin SD, Prus AJ, Pehrson AL, Porter JH (2005) Serotonin receptor mechanisms mediate the discriminative stimulus properties of the atypical antipsychotic clozapine in C57BL/6 mice. Psychopharmacology (Berl) 180:49–56
Plakke B, Ng CW, Poremba A (2008) Scopolamine impairs auditory delayed matching to sample performance in monkeys. Neurosci Lett. 438(1):126–130
Porter JH, Varvel SA, Vann RE, Philibin SD, Wise LE (2000) Clozapine discrimination with a low training dose distinguishes atypical from typical antipsychotic drugs in rats. Psychopharmacology (Berl) 149(2):189–93
Porter JH, Prus AJ, Vann RE, Varvel SA (2005) Discriminative stimulus properties of the atypical antipsychotic clozapine and the typical antipsychotic chlorpromazine in a three-choice drug discrimination procedure in rats. Psychopharmacology 178:67–77
Prus AJ, Baker LE, Meltzer HY (2004) Discriminative stimulus properties of 1.25 mg/kg and 5.0 mg/kg doses of clozapine in rats: examination of the role of dopamine, serotonin and muscarinic receptor mechanisms. Pharmacol Biochem Behav 77:199–208
Prus AJ, Philibin SD, Pehrson AL, Stephens CL, Cooper RN, Wise LE, Porter JH (2005) Generalization testing with atypical and typical antipsychotic drugs in rats trained to discriminate 5.0 mg/kg clozapine from vehicle in a two-choice drug discrimination task. Drug Dev Res 64:55–66
Prus AJ, Philibin SD, Pehrson AL, Porter JH (2006) Discriminative stimulus properties of the atypical antipsychotic drug clozapine in rats trained to discriminate 1.25 mg/kg clozapine vs. 5.0 mg/kg clozapine vs. vehicle. Behav Pharmacol 17:185–194
Riedel W, Hogervorst E, Leboux R, Verhey F, Van Praag J, Jolles J (1995) Caffeine attenuates scopolamine-induced memory impairments in humans. Psychopharmacology. 122(2):158–68
Scholz E, Dichgans J (1985) Treatment of drug-induced exogenous psychosis in parkinsonism with clozapine and fluperlapine. Eur Arch Psychiatry Neurol Sci 235:60–64
Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE (1996) Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology 124:57–73
Tamminga CA, Eamma J, Ibraham H, Jain S, Taylor E, Vanover KE, Hacksell U, Brann M, van Kammen DP (2006) ACP-104 tolerability, safety and pharmacokinetics: single rising dose study. Society for Neuroscience, Atlanta, GA
Wahlbeck K, Cheine MV, Essali A (2008) Clozapine versus typical neuroleptic medication for schizophrenia (review). The Cochrane Collaboration, issue 2, pp 1–88. Wiley, New York. http://www.thecochranelibrary.com/
Weiner DM, Meltzer HY, Veinbergs I, Donohue EM, Spalding TA, Smith TT, Mohell N, Harvey SC, Lameh J, Nash N, Vanover KE, Olsson R, Jayathilake K, Lee M, Levey AI, Hacksell U, Burstein ES, Davis RE, Brann MR (2004) The role of M1 muscarinic receptor agonism of N-desmethylclozapine in the unique clinical effects of clozapine. Psychopharmacology 177:207–216
Young CR, Longhurst JG, Bowers Jr MB, Mazure CM (1997) The expanding indications for clozapine. Exp Clin Psychopharmacol 5(3):216–234
Acknowledgments
A portion of this research was supported by NIH Grant 5 F31 MH070154-03 to A.L.P. and NIH Grant 1 F31 GM070974-03 to S.D.P.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prus, A.J., Pehrson, A.L., Philibin, S.D. et al. The role of M1 muscarinic cholinergic receptors in the discriminative stimulus properties of N-desmethylclozapine and the atypical antipsychotic drug clozapine in rats. Psychopharmacology 203, 295–301 (2009). https://doi.org/10.1007/s00213-008-1262-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1262-0