Abstract
Rationale
Benzodiazepine treatment can result in dependence as evidenced by signs of withdrawal upon discontinuation of use.
Objective
Positive GABAA receptor modulators were examined for their capacity to attenuate flumazenil-like discriminative stimulus effects (i.e., withdrawal) that emerge upon discontinuation of chronic benzodiazepine treatment.
Methods
Rhesus monkeys receiving chronic diazepam (5.6 mg−1 kg−1 24 h−1 p.o.) discriminated flumazenil (0.1 mg/kg s.c.) from vehicle.
Results
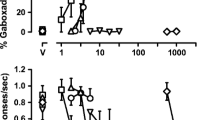
Upon discontinuation of diazepam treatment, responding switched from the vehicle to the flumazenil lever, although at different times among monkeys. The shorter-acting benzodiazepine lorazepam (3.2 mg−1 kg−1 8 h−1) was substituted for diazepam and, 11 h after lorazepam, monkeys consistently responded on the flumazenil lever. Flumazenil-lever responding after acute lorazepam deprivation was attenuated not only by benzodiazepines (lorazepam and midazolam) but also by positive GABAA receptor modulators acting at neuroactive steroid (pregnanolone and alfaxalone) and barbiturate sites (pentobarbital). Deprivation-induced responding on the flumazenil lever was not attenuated by low efficacy positive GABAA modulators (bretazenil and L-838,417) or non-GABAA receptor ligands (ketamine and cocaine). Neuroactive steroids were relatively more potent than other positive GABAA receptor modulators in attenuating deprivation-induced flumazenil-lever responding, as compared to their relative potency in monkeys discriminating midazolam and otherwise not receiving benzodiazepine treatment.
Conclusions
These results suggest that positive GABAA receptor modulators acting at different sites attenuate withdrawal induced by discontinuation of benzodiazepine treatment, consistent with previous studies suggesting that the same compounds attenuate flumazenil-precipitated withdrawal. Differences in the relative potency of positive modulators as a function of acute versus chronic benzodiazepine treatment suggest that neuroactive steroids, in particular, are especially potent in benzodiazepine-dependent animals.





Similar content being viewed by others
References
Ashton H (2005) The diagnosis and management of benzodiazepine dependence. Curr Opin Psychiatry 18:249–255
Ator NA, Griffiths RR (1997) Selectivity in the generalization profile in baboons trained to discriminate lorazepam: benzodiazepines, barbiturates and other sedative/anxiolytics. J Pharmacol Exp Ther 282:1442–1457
Curry SH, Whelpton R, Nicholson AN, Wright CM (1977) Behavioural and pharmacokinetic studies in the monkey (Macaca mulatta) with diazepam, nordiazepam and related 1,4-benzodiazepines. Br J Pharmacol 61:325–330
de la Garza R, Johanson CE (1987) Discriminative stimulus properties of intragastrically administered d-amphetamine and pentobarbital in rhesus monkeys. J Pharmacol Exp Ther 243:955–962
Devaud LL, Purdy RH, Finn DA, Morrow AL (1996) Sensitization of gamma-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharmacol Exp Ther 278:510–517
Emmett-Oglesby MW, Rowan GA (1991) Drug discrimination used to study drug withdrawal. In: Glennon RA, Jarbe TUC, Frankenheim J (eds) Drug discrimination: applications to drug abuse research. US Government Printing Office, Washington, DC. NIDA Res Monogr 116:337–357
Facklam M, Schoch P, Haefely WE (1992) Relationship between benzodiazepine receptor occupancy and potentiation of gamma-aminobutyric acid-stimulated chloride flux in vitro of four ligands of differing intrinsic efficacies. J Pharmacol Exp Ther 261:1106–1112
Gerak LR, France CP (1999) Discriminative stimulus effects of flumazenil in untreated and in diazepam-treated rhesus monkeys. Psychopharmacology 146:252–261
Griffiths RR, Evans SM, Guarino JJ, Roache JD, Furman WR, Liebson I, Schwam EM (1993) Intravenous flumazenil following acute and repeated exposure to lorazepam in healthy volunteers: antagonism and precipitated withdrawal. J Pharmacol Exp Ther 265:1163–1174
Kenakin T (1997) Pharmacologic analysis of drug-receptor interaction. Lippincott-Raven, Philadelphia, PA
Lukas SE, Griffiths RR (1982) Precipitated withdrawal by a benzodiazepine receptor antagonist (Ro 15-1788) after 7 days of diazepam. Science 217:1161–1163
Maksay G, Tegyey Z, Simonyi M (1991) Central benzodiazepine receptors: in vitro efficacies and potencies of 3-substituted 1,4-benzodiazepine stereoisomers. Mol Pharmacol 39:725–732
Martin JR, Jenck F, Moreau JL (1995) Comparison of benzodiazepine receptor ligands with partial agonistic, antagonistic or partial inverse agonistic properties in precipitating withdrawal in squirrel monkeys. J Pharmacol Exp Ther 275:405–411
McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P et al (2000) Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor∝1 subtype. Nat Neurosci 3:587–592
McMahon LR, France CP (2003) Discriminative stimulus effects of positive GABAA modulators and other anxiolytics, sedatives, and anticonvulsants in untreated and diazepam-treated monkeys. J Pharmacol Exp Ther 304:109–120
McMahon LR, France CP (2006) Differential behavioral effects of low efficacy positive GABA(A) modulators in combination with benzodiazepines and a neuroactive steroid in rhesus monkeys. Br J Pharmacol 147:260–268
McMahon LR, Gerak LR, France CP (2001) Potency of positive GABAA modulators to substitute for a midazolam discriminative stimulus in untreated monkeys does not predict potency to attenuate a flumazenil discriminative stimulus in diazepam treated monkeys. J Pharmacol Exp Ther 298:1227–1235
Mehta AK, Ticku MK (1999) An update on GABAA receptors. Brain Res Brain Res Rev 29:196–217
Mintzer MZ, Griffiths RR (2005) Flumazenil-precipitated withdrawal in healthy volunteers following repeated diazepam exposure. Psychopharmacology 178:259–267
Morrow AL, Van Doren MJ, Penland SN, Matthews DB (2001) The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev 37:98–109
Pesold C, Caruncho HJ, Impagnatiello F, Berg MJ, Fritschy JM, Guidotti A, Costa E (1997) Tolerance to diazepam and changes in GABAA receptor subunit expression in rat neocortical areas. Neuroscience 79:477–487
Puia G, Santi M, Vincini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E (1990) Neurosteroids act on recombinant human GABAA receptors. Neuron 4:759–765
Sell SL, McMahon LR, France CP (2003) Relative efficacy of buprenorphine, nalbuphine and morphine in opioid-treated rhesus monkeys discriminating naltrexone. J Pharmacol Exp Ther 306:1167–1173
Smith AJ, Alder L, Silk J, Adkins C, Fletcher AE, Scales T, Kerby J, Marshall G, Wafford KA, McKernan RM, Atack JR (2001) Effect of subunit on allosteric modulation of ion channel function in stably expressed human recombinant-aminobutyric acidA receptors determined using 36Cl ion flux. Mol Pharmacol 59:1108–1118
Tallarida RJ (2000) Drug synergism and dose-effect data analysis. CRC, Boca Raton, FL
Acknowledgment
This study was supported by US Public Health Service Grant DA09157 and a Senior Scientist Award to C.P.F. (K05 DA17918). The authors thank C. Cruz, B. Harrington, M. Hernandez, D. Logan, R. Luna, and K. Stone for providing technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McMahon, L.R., Javors, M.A. & France, C.P. Changes in relative potency among positive GABAA receptor modulators upon discontinuation of chronic benzodiazepine treatment in rhesus monkeys. Psychopharmacology 192, 135–145 (2007). https://doi.org/10.1007/s00213-006-0692-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0692-9