Abstract
Rationale
Early life exposure to stress and to GABAA receptor modulators have well-defined and persistent behavioral effects. A single neonatal injection of the GABAergic neurosteroid allopregnanolone (3α-hydroxy,5α-pregnane-20-one, 10 mg/kg, i.p.) alters the localization of prefrontal cortex (PFC) interneurons in adulthood. Such displacement could result in disinhibited behavior associated with impaired development of the mesocortical dopamine system.
Objectives
To determine if there is a critical window in which allopregnanolone levels may impact the development and mature function of the mesocorticolimbic circuitry.
Methods
Behavioral measures, including prepulse inhibition (PPI) and total locomotor activity, after amphetamine exposure were assessed at postnatal day 20 (P20) (prepuberty), P40 (puberty), P60 (postpuberty), and P80 (adulthood) in animals previously exposed to allopregnanolone (10 mg/kg) on P2 and P5. PFC tyrosine hydroxylase immunoreactivity was stereologically measured.
Results
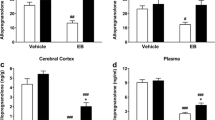
P2 administration of allopregnanolone resulted in an increased locomotor response to amphetamine (14, 28% on P20 and P80, respectively) and reduced PPI (28, 22% on P20 and P80, respectively) at P20 and P80, whereas allopregnanolone administration on P5 increased locomotor response to amphetamine (20%) and reduced PPI (37%) at P80. Clozapine (7.5 mg/kg) pretreatment reversed the PPI deficit in P2-exposed animals. The total length of tyrosine hydroxylase immunopositive fibers in PFC was not altered by neonatal neurosteroid exposure, but more fibers were located in layers V/VI vs I–III.
Conclusions
Altering neonatal allopregnanolone levels disrupts PFC-dependent behavior, indicating that allopregnanolone might be important for normal PFC circuitry development. The temporal exposure differences (P2 vs P5) and ontological-dependent effects (P20 and P80, but not P40 or P60) suggest critical windows of vulnerability to neurosteroid insult across development.




Similar content being viewed by others
References
Adler LE, Olincy A, Cawthra EM, McRae KA, Harris JG, Nagamoto HT, Waldo MC, Hall MH, Bowles A, Woodward L, Ross RG, Freedman R (2004) Varied effects of atypical neuroleptics on P50 auditory gating in schizophrenia patients. Am J Psychiatry 161:1822–1828
Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA (1999) Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry 156:1580–1589
An JJ, Bae MH, Cho SR, Lee SH, Choi SH, Lee BH, Shin HS, Kim YN, Park KW, Borrelli E, Baik JH (2004) Altered GABAergic neurotransmission in mice lacking dopamine D2 receptors. Mol Cell Neurosci 25:732–741
Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH (2000) Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse 37:167–169
Antonopoulos J, Dori I, Dinopoulos A, Chiotelli M, Parnavelas JG (2002) Postnatal development of the dopaminergic system of the striatum in the rat. Neuroscience 110:245–256
Bakshi VP, Geyer MA (1999) Ontogeny of isolation rearing-induced deficits in sensorimotor gating in rats. Physiol Behav 67:385–392
Benes FM, Berretta S (2001) GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 25:1–27
Billiards SS, Walker DW, Canny BJ, Hirst JJ (2002) Endotoxin increases sleep and brain allopregnanolone concentrations in newborn lambs. Pediatr Res 52:892–899
Brixey SN, Gallagher BJ III, McFalls JA Jr, Parmelee LF (1993) Gestational and neonatal factors in the etiology of schizophrenia. J Clin Psychol 49:447–456
Collins SL, Montano R, Izenwasser S (2004) Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Brain Res Dev Brain Res 153:175–187
Ellenbroek BA, Budde S, Cools AR (1996) Prepulse inhibition and latent inhibition: the role of dopamine in the medial prefrontal cortex. Neuroscience 75:535–542
Gilmore JH, Jarskog LF (1997) Exposure to infection and brain development: cytokines in the pathogenesis of schizophrenia. Schizophr Res 24:365–367
Gizerian SS, Morrow AL, Lieberman JA, Grobin AC (2004) Neonatal neurosteroid administration alters parvalbumin expression and neuron number in medial dorsal thalamus of adult rats. Brain Res 1012:66–74
Griffin LD, Gong W, Verot L, Mellon SH (2004) Niemann–Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med 10:704–711
Grobin AC, Heenan EJ, Lieberman JA, Morrow AL (2003) Perinatal neurosteroid levels influence GABAergic interneuron localization in adult rat prefrontal cortex. J Neurosci 23:1832–1839
Groves PM, Thompson RF (1970) Habituation: a dual-process theory. Psychol Rev 77:419–450
Gruen RJ, Deutch AY, Roth RH (1990) Perinatal diazepam exposure: alterations in exploratory behavior and mesolimbic dopamine turnover. Pharmacol Biochem Behav 36:169–175
Gruen RJ, Wenberg K, Selim M, Friedhoff AJ, Bradberry CW (1996) Novelty-associated locomotion: correlation with cortical and sub-cortical GABAA receptor binding. Eur J Pharmacol 309:115–120
Japha K, Koch M (1999) Picrotoxin in the medial prefrontal cortex impairs sensorimotor gating in rats: reversal by haloperidol. Psychopharmacology (Berl) 144:347–354
Kellogg CK (1999) Sex differences in long-term consequences of prenatal diazepam exposure: possible underlying mechanisms. Pharmacol Biochem Behav 64:673–680
Kumari V, Soni W, Sharma T (1999) Normalization of information processing deficits in schizophrenia with clozapine. Am J Psychiatry 156:1046–1051
Lindley SE, Bengoechea TG, Schatzberg AF, Wong DL (1999) Glucocorticoid effects on mesotelencephalic dopamine neurotransmission. Neuropsychopharmacology 21:399–407
Marcelis M, van Os J, Sham P, Jones P, Gilvarry C, Cannon M, McKenzie K, Murray R (1998) Obstetric complications and familial morbid risk of psychiatric disorders. Am J Med Genet 81:29–36
Maric D, Liu QY, Maric I, Chaudry S, Chang YH, Smith SV, Sieghart W, Fritschy JM, Barker JL (2001) GABA expression dominates neuronal lineage progression in the embryonic rat neocortex and facilitates neurite outgrowth via GABA(A) autoreceptor/Cl− channels. J Neurosci 21:2343–2360
Mirescu C, Peters JD, Gould E (2004) Early life experience alters response of adult neurogenesis to stress. Nat Neurosci 7:841–846
Owens DF, Liu X, Kriegstein AR (1999) Changing properties of GABA(A) receptor-mediated signaling during early neocortical development. J Neurophysiol 82:570–583
Park M, Kitahama K, Geffard M, Maeda T (2000) Postnatal development of the dopaminergic neurons in the rat mesencephalon. Brain Dev 22(Suppl 1):S38–S44
Paylor R, Crawley JN (1997) Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl) 132:169–180
Pierce RC, Kalivas PW (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 25:192–216
Purdy RH, Morrow AL, Moore PH Jr, Paul SM (1991) Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A 88:4553–4557
Swerdlow NR, Varty GB, Geyer MA (1998) Discrepant findings of clozapine effects on prepulse inhibition of startle: is it the route or the rat? Neuropsychopharmacology 18:50–56
Swerdlow NR, Geyer MA, Braff DL (2001) Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 156:194–215
Swerdlow NR, Pitcher L, Noh HR, Shoemaker JM (2002) Startle gating in rats is disrupted by chemical inactivation but not D2 stimulation of the dorsomedial thalamus. Brain Res 953:246–254
Toufexis DJ, Davis C, Hammond A, Davis M (2004) Progesterone attenuates corticotropin-releasing factor-enhanced but not fear-potentiated startle via the activity of its neuroactive metabolite, allopregnanolone. J Neurosci 24:10280–10287
van den Buuse M, Morris M, Chavez C, Martin S, Wang J (2004) Effect of adrenalectomy and corticosterone replacement on prepulse inhibition and locomotor activity in mice. Br J Pharmacol 142:543–550
Van Eden CG (1986) Development of connections between the mediodorsal nucleus of the thalamus and the prefrontal cortex in the rat. J Comp Neurol 244:349–359
Van Eden CG, Uylings HB (1985a) Cytoarchitectonic development of the prefrontal cortex in the rat. J Comp Neurol 241:253–267
Van Eden CG, Uylings HB (1985b) Postnatal cytoarchetectonic development of the prefrontal cortex in the rat. J Comp Neurol 241:253–267
Varshavskaya VM, Ivanova ON, Yakimovskii AF (2004) Motor behavior in rats after separate and combined administration of GABAergic agents into the neostriatum. Neurosci Behav Physiol 34:293–298
Wise SP, Jones EG (1978) Development studies of thalamocortical and commissural connections in the rat somatic sensory cortex. J Comp Neurol 178:187–208
Wolf ME (1998) The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol 54:679–720
Zavitsanou K, Cranney J, Richardson R (1999) Dopamine antagonists in the orbital prefrontal cortex reduce prepulse inhibition of the acoustic startle reflex in the rat. Pharmacol Biochem Behav 63:55–61
Zimmerberg B, McDonald BC (1996) Prenatal alcohol exposure influences the effects of neuroactive steroids on separation-induced ultrasonic vocalizations in rat pups. Pharmacol Biochem Behav 55:541–547
Acknowledgements
The authors would like to thank Antonio Perez of the University of North Carolina Neurodevelopmental Research Center Mouse Phenotyping Core for technical assistance. This work was funded by a Stanley Foundation Stanley Scholars Grant, MH065470 (A.C. Grobin), and the Silvio O. Conte Center for the Neuroscience of Mental Disorders.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gizerian, S.S., Moy, S.S., Lieberman, J.A. et al. Neonatal neurosteroid administration results in development-specific alterations in prepulse inhibition and locomotor activity. Psychopharmacology 186, 334–342 (2006). https://doi.org/10.1007/s00213-006-0360-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0360-0