Abstract
Rationale
The brainstem pedunculopontine tegmental nucleus (PPTg) is proposed to mediate hypothalamic self-stimulation reward via cholinergic activation of the ventral tegmental area (VTA). However, to date there is little direct evidence to support this hypothesis.
Objectives
To further study the role of PPTg in hypothalamic self-stimulation reward.
Methods
By using in vivo microdialysis, the levels of extracellular acetylcholine (ACh) in the PPTg and VTA were detected during lateral hypothalamic (LH) self-stimulation in rats. Rate–frequency curve shift procedure was used to evaluate the effects of nonselective muscarinic antagonist scopolamine (1∼100 μg/μl) and nicotinic antagonist mecamylamine (5∼100 μg/μl) microinjected into the PPTg on the rewarding efficacy of LH self-stimulation. Subsequently, the drugs were injected into the PPTg, and the extracellular ACh in the VTA was measured.
Results
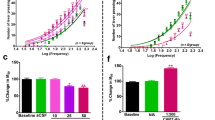
LH self-stimulation produced a concurrent ACh release in the PPTg and VTA. Intra-PPTg injection of scopolamine (100 μg/μl) significantly reduced the frequency threshold for LH self-stimulation reward, but nicotinic antagonist mecamylamine did not shift the threshold. However, mecamylamine (10, 25 μg/μl) injected into the PPTg robustly diminished the nicotine-potentiated LH self-stimulation reward. The extracellular ACh in the VTA was dramatically increased by intra-PPTg scopolamine (10, 100 μg/μl), but not by mecamylamine.
Conclusions
Results confirm that PPTg plays an important role in brain stimulation reward by modulating the cholinergic activity of the VTA. The PPTg muscarinic receptors contribute to an inhibitory modulation of reward effects by self-stimulation, whereas nicotinic receptors seem to be more involved in nicotine potentiation of brain stimulation reward.





Similar content being viewed by others
References
Alderson HL, Latimer MP, Blaha CD, Phillips AG, Winn P (2004) An examination of d-amphetamine self-administration in pedunculopontine tegmental nucleus-lesioned rats. Neuroscience 125:349–358
Arnold HM, Nelson CL, Sarter M, Bruno JP (2003) Sensitization of cortical acetylcholine release by repeated administration of nicotine in rats. Psychopharmacology (Berl) 165:346–358
Blaha CD, Allen LF, Das S, Inglis WL, Latimer MP, Vincent SR, Winn P (1996) Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned, and laterodorsal tegmental nucleus-lesioned rats. J Neurosci 16:714–722
Chapman CA, Yeomans JS, Blaha CD, Blackburn JR (1997) Increased striatal dopamine efflux follows scopolamine administered systemically or to the tegmental pedunculopontine nucleus. Neuroscience 76:177–186
Corrigall WA, Coen KM, Adamson KL, Chow BLC (1999) Manipulations of mu-opioid and nicotinic cholinergic receptors in the pontine tegmental region alter cocaine self-administration in rats. Psychopharmacology (Berl) 145:412–417
Corrigall WA, Coen KM, Zhang J, Adamson KL (2001) GABA mechanisms in the pedunculopontine tegmental nucleus influence particular aspects of nicotine self-administration selectively in the rat. Psychopharmacology (Berl) 158:190–197
Corrigall WA, Coen KM, Zhang J, Adamson KL (2002) Pharmacological manipulations of the pedunculopontine tegmental nucleus in the rat reduce self-administration of both nicotine and cocaine. Psychopharmacology (Berl) 160:198–205
Diederich K, Koch M (2005) Role of the pedunculopontine tegmental nucleus in sensorimotor gating and reward-related behavior in rats. Psychopharmacology (Berl) 179:402–408
Floresco SB, West AR, Ash B, Moore H, Grace AA (2003) Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6:968–973
Forster GL, Blaha CD (2000) Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci 12:3596–3604
Forster GL, Blaha CD (2003) Pedunculopontine tegmental stimulation evokes striatal dopamine efflux by activation of acetylcholine and glutamate receptors in the midbrain and pons of the rat. Eur J Neurosci 17:751–762
Garzon M, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM (1999) Cholinergic axon terminals in the ventral tegmental area target a subpopulation of neurons expressing low levels of the dopamine transporter. J Comp Neurol 410:197–210
Grillner P, Berretta N, Bernardi G, Svensson TH, Mercuri NB (2000) Muscarinic receptors depress GABAergic synaptic transmission in rat midbrain dopamine neurons. Neuroscience 96:299–307
Harrison AA, Gasparini F, Markou A (2002) Nicotine potentiation of brain stimulation reward reversed by DHβE and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 160:56–66
Hildebrand BE, Panagis G, Svensson TH, Nomikos GG (1999) Behavioral and biochemical manifestations of mecamylamine-precipitated nicotine withdrawal in the rat: role of nicotinic receptors in the ventral tegmental area. Neuropsychopharmacology 21:561–574
Ikemoto S, Wise RA (2004) Mapping of chemical trigger zones for reward. Neuropharmacology 47:190–201
Ivanova S, Greenshaw AJ (1997) Nicotine-induced decreases in VTA electrical self-stimulation thresholds: blockade by haloperidol and mecamylamine but not scopolamine or ondansetron. Psychopharmacology (Berl) 134:187–192
Kelley AE (2002) Nicotinic receptors: addiction’s smoking gun? Nat Neurosci 8:447–449
Kippin TE, van der Kooy D (2003) Excitotoxic lesions of the tegmental pedunculopontine nucleus impair copulation in naive male rats and block the rewarding effects of copulation in experienced male rats. Eur J Neurosci 18:2581–2591
Lanca AJ, Adamson KL, Coen KM, Chow BLC, Corrigall WA (1999) The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: a correlative neuroanatomical and behavioral study. Neuroscience 96:735–742
Lanca AJ, Sanelli TR, Corrigall WA (2000) Nicotine-induced Fos expression in the pedunculopontine messencephalic tegmentum in the rat. Neuropharmacology 39:2808–2817
Mena-Segovia J, Bolam JP, Magill PJ (2004) Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci 27:585–588
Miller AD, Blaha CD (2004) Nigrostriatal dopamine release modulated by mesopontine muscarinic receptors. Neuroreport 15:1805–1808
Miller AD, Blaha CD (2005) Midbrain muscarinic receptor mechanisms underlying regulation of mesoaccumbens and nigrostriatal dopaminergic transmission in the rat. Eur J Neurosci 21:1837–1846
Nakahara D, Ishida Y, Nakamura M, Furuno N, Nishimori T (2001) Intracranial self-stimulation induces Fos expression in GABAergic neurons in the rat mesopontine tegmentum. Neuroscience 106:633–641
Nisell M, Nomikos GG, Svensson TH (1994) Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse 16:36–44
Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK (1995) Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci 15:5859–5869
Olmstead MC, Munn EM, Franklin KBJ, Wise RA (1998) Effects of pedunculopontine tegmental nucleus lesions on responding for intravenous heroin under different schedules of reinforcement. J Neurosci 18:5035–5044
Pahapill PA, Lozano AM (2000) The pedunculopontine nucleus and Parkinson’s disease. Brain 123:1767–1783
Panagis G, Kastellakis A, Spyraki C, Nomikos G (2000) Effects of methyllycaconitine (MLA), an α7 nicotinic receptor antagonist, on nicotine- and cocaine-induced potentiation of brain stimulation reward. Psychopharmacology (Berl) 149:388–396
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic, San Diego
Picciotto MR, Corrigall WA (2002) Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci 22:3336–3341
Rada PV, Mark GP, Yeomans JJ, Hoebel BG (2000) Acetylcholine release in ventral tegmental area by hypothalamic self-stimulation, eating and drinking. Pharmacol Biochem Behav 65:375–379
Redgrave P, Horrell RI (1976) Potentiation of central reward by localized perfusion of acetylcholine and 6-hydroxytryptamine. Nature 262:305–307
Reid RT, Lloyd GK, Rao TS (1999) Pharmacological characterization of nicotine-induced acetylcholine release in the rat hippocampus in vivo: evidence for a permissive dopamine synapse. Br J Pharmacol 127:1486–1494
Roth MT, Fleegal MA, Lydic R, Baghdoyan HA (1996) Pontine acetylcholine release is regulated by muscarinic autoreceptors. Neuroreport 7:3069–3072
Ryan RE, Loiacono RE (2000) Nicotinic receptor subunit mRNA in the thalamus of the rat: relevance to schizophrenia? Neuroreport 11:3693–3698
Satoh K, Fibiger HC (1986) Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J Comp Neurol 253:277–302
Schilstrom B, Svensson HM, Svensson TH, Nomikos GG (1998) Nicotine and food induced dopamine release in the nucleus accumbens of the rat: putative role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroscience 85:1005–1009
Semba K, Fibiger HC (1992) Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol 323:387–410
Steininger TL, Rye DB, Wainer BH (1992) Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. Retrograde tracing studies. J Comp Neurol 321:515–543
Summers KL, Giacobini E (1995) Effects of local and repeated systemic administration of (−) nicotine on extracellular levels of acetylcholine, norepinephrine, dopamine, and serotonin in rat cortex. Neurochem Res 20:753–759
Summers KL, Cuadra G, Naritoku D, Giacobini E (1994) Effects of nicotine on levels of acetylcholine and biogenic amines in rat cortex. Drug Dev Res 31:108–119
Tani Y, Saito K, Imoto M, Ohno T (1998) Pharmacological characterization of nicotinic receptor-mediated acetylcholine release in rat brain—an in vivo microdialysis study. Eur J Pharmacol 351:181–188
Tzschentke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56:613–672
Vilaro MT, Palacios JM, Mengod G (1994) Multiplicity of muscarinic autoreceptor subtypes? Comparison of the distribution of cholinergic cells and cells containing mRNA for five subtypes of muscarinic receptors in the rat brain. Brain Res Mol Brain Res 21:30–46
Waraczynski M, Perkins M (1998) Lesions of pontomesencephalic cholinergic nuclei do not substantially disrupt the reward value of medial forebrain bundle stimulation. Brain Res 800:154–169
Wise RA (2002) Brain reward circuitry: insights from unsensed incentives. Neuron 36:229–240
Wise RA, Marcangione C, Bauco P (1998) Blockade of the reward-potentiating effects of nicotine on lateral hypothalamic brain stimulation by chlorisondamine. Synapse 29:72–79
Yeomans JS (1989) Two substrates for medial forebrain bundle self-stimulation: myelinated axons and dopamine axons. Neurosci Biobehav Rev 13:91–98
Yeomans JS, Baptista M (1997) Both nicotine and muscarinic receptors in ventral tegmental area contribute to brain-stimulation reward. Pharmacol Biochem Behav 57:915–921
Yeomans JS, Mathur A, Tampakeras M (1993) Rewarding brain stimulation: role of tegmental cholinergic neurons that activate dopamine neurons. Behav Neurosci 107:1077–1087
Yeomans J, Forster G, Blaha C (2001) M5 muscarinic receptors are needed for slow activation of dopamine neurons and for rewarding brain stimulation. Life Sci 68:2449–2456
You ZB, Chen YQ, Wise RA (2001) Dopamine and glutamate release in the nucleus accumbens and ventral tegmental area of rat following lateral hypothalamic self-stimulation. Neuroscience 107:629–639
Acknowledgements
We thank Dr. Gregory V.G. O’Dowd for editing the manuscript. This research was supported in part by a Grant-in-Aid for Scientific Research, the Ministry of Education, Science, Sports, and Culture of Japan and by the Smoking Research Foundation Grant for Biomedical Research (Japan).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J., Nakamura, M., Kawamura, T. et al. Roles of pedunculopontine tegmental cholinergic receptors in brain stimulation reward in the rat. Psychopharmacology 184, 514–522 (2006). https://doi.org/10.1007/s00213-005-0252-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0252-8