Abstract
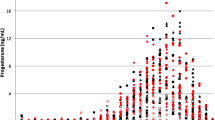
Rationale: The endogenous GABAergic neuroactive steroid 3α-hydroxy-5α-pregnan-20-one (3α,5α-THP, allopregnanolone) has been proposed to contribute to ethanol actions. Humans synthesize 3α,5α-THP, but its role in response to systemic administration of ethanol is unclear. Objective: The present study aims to determine the effect of a moderate dose of ethanol on progesterone and 3α,5α-THP concentrations in plasma samples of healthy male and female subjects and to determine if these levels are related to the subjective effects of ethanol. Females were tested in both the follicular and luteal phases of the menstrual cycle. Methods: Healthy men (N=9) and women (N=12) aged 21–35 participated in the study. Men participated in two sessions on which they received ethanol (0.8 g/kg) or placebo. Women participated in four sessions on which they received ethanol (0.7 g/kg) or placebo during the follicular and luteal phases of their cycle. Subjective states and mood were measured by standardized self-report questionnaires and a measure of psychomotor performance. Steroid levels (progesterone, 3α,5α-THP, estradiol, and cortisol) were measured in plasma samples by radioimmunoassay. Results: Ethanol significantly increased plasma levels of progesterone, but not 3α,5α-THP-like immunoreactivity, in women in the luteal phase. Ethanol had no effect on progesterone or 3α,5α-THP-like immunoreactivity levels in women in the follicular phase or in men, and it did not increase cortisol in men or women. Ethanol also did not affect estradiol in men or women. Conclusions: 3α,5α-THP-like immunoreactivity levels in human plasma are not increased following moderate ethanol consumption, suggesting that circulating levels of progesterone or its tetrahydro-reduced metabolites do not play a major role in ethanol action. However, the possibility remains that ethanol increases endogenous brain production of GABAergic neurosteroids without affecting plasma levels. Moreover, humans synthesize 5β-reduced GABAergic steroids, and levels of these steroids may be altered in plasma or brain.



Similar content being viewed by others
References
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. APA Press, Washington, DC
Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R, Gessa GL (1999) Ethanol markedly increases “GABAergic” neurosteroids in alcohol-preferring rats. Eur J Pharmacol 384:R1–R2
Brick J, Nathan PE, Westrick E, Frankenstein W, Shapiro A (1986) The effect of menstrual cycle on blood alcohol levels and behavior. J Stud Alcohol 47:472–477
Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA (1987) Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci 231:359–369
Dai X, Thavundayil J, Gianoulakis C (2002) Response of the hypothalamic–pituitary–adrenal axis to stress in the absence and presence of ethanol in subjects at high and low risk of alcoholism. Neuropsychopharmacology 27:442–452
Derogatis LR, Melisaratos N (1983) The Brief Symptom Inventory: an introductory report. Psychol Med 13:595–605
Foltin RW, Fischman MW (1991) Methods for the assessment of abuse liability of psychomotor stimulants and anorectic agents in humans. Br J Addict 86:1633–1640
Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M (1998) Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab 83(6):2099–2103
Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL (2001) Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry 49:788–797
Green KL, Azarov AV, Szeliga KT, Purdy RH, Grant KA (1999) The influence of menstrual cycle phase on sensitivity to ethanol-like discriminative stimulus effects of GABAA-positive modulators. Pharmacol Biochem Behav 64:379–383
Griffin LD, Mellon SH (2001) Biosynthesis of the neurosteroid 3 alpha-hydroxy-4-pregnen-20-one (3 alpha hp), a specific inhibitor of FSH release. Endocrinology 142:4617–4622
Griffin JE, Ojeda SR (1996) Textbook of endocrine physiology. Oxford University Press, New York
Hill M, Popov P, Havlikova H, Kancheva L, Vrbikova J, Meloun M, Kancheva R, Cibula D, Pouzar V, Cerny I, Starka L (2005) Reinstatement of serum pregnanolone isomers and progesterone during alcohol detoxification therapy in premenopausal women. Alcohol Clin Exp Res 29:1010–1017
Hirani K, Khisti RT, Chopde CT (2002) Behavioral action of ethanol in Porsolt's forced swim test: modulation by 3α-hydroxy-5α-pregnan-20-one. Neuropharmacology 43:1339–1350
Hirani K, Sharma AN, Jain NS, Ugale RR, Chopde CT (2005) Evaluation of GABAergic neuroactive steroid 3alpha-hydroxy-5alpha-pregnane-20-one as a neurobiological substrate for the anti-anxiety effect of ethanol in rats. Psychopharmacology 180:267–278
Holdstock L, de Wit H (1998) Individual differences in the biphasic effects of ethanol. Alcohol Clin Exp Res 22:1903–1911
Holdstock L, de Wit H (1999) Individual differences in subjective responses to ethanol and triazolam. Behav Pharmacol 10:283–295
Holdstock L, de Wit H (2000) Effects of ethanol at four phases of the menstrual cycle. Psychopharmacology 150:374–382
Janak PH, Redfern JEM, Samson HH (1998) The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol Clin Exp Res 22:1106–1112
Janis GC, Devaud LL, Mitsuyama H, Morrow AL (1998) Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in male and female rats. Alcohol Clin Exp Res 22:2055–2061
Khisti RT, VanDoren MJ, O'Buckley TK, Morrow AL (2003) Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates ethanol-induced loss of righting reflex in rats. Brain Res 980:255–265
Khisti RT, Boyd KN, Kumar S, Morrow AL (2005) Systemic ethanol administration elevates deoxycorticosterone levels and chronic ethanol exposure attenuates this response. Brain Res 1049:104–111
King AC, Houle T, de Wit H, Holdstock L, Schuster A (2002) Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res 26:827–835
Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM (1986) Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232:1004–1007
Martin WR, Sloan JW, Sapira JD, Jasinski DR (1971) Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther 12:245–258
Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM (1993) Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res 17:140–146
Matthews DB, Morrow AL, Tokunaga S, McDaniel JR (2002) Acute ethanol administration and acute allopregnanolone administration impair spatial memory in the Morris water task. Alcohol Clin Exp Res 26:1747–1751
McCaul ME, Wand GS, Stauffer R, Lee SM, Rohde CA (2001) Naltrexone dampens ethanol-induced cardiovascular and hypothalamic–pituitary–adrenal axis activation. Neuropsychopharmacology 25:537–547
McNamee B, Grant J, Ratcliffe J, Ratcliffe W, Oliver J (1979) Lack of effect of alcohol on pituitary–gonadal hormones in women. Br J Addict Alcohol Other Drugs 74:316–317
Mendelson JH, Mello NK, Teoh SK, Ellingboe J (1989) Alcohol effects on luteinizing hormone releasing hormone-stimulated anterior pituitary and gonadal hormones in women. J Pharmacol Exp Ther 250:902–909
Morrow AL, Suzdak PD, Paul SM (1987) Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol 142:483–485
Morrow AL, Pace JR, Purdy RH, Paul SM (1990) Characterization of steroid interactions with γ-aminobutyric acid receptor-gated chloride ion channels: evidence for multiple steroid recognition sites. Mol Pharmacol 37:263–270
Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, Grant KA (1999) Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcohol Clin Exp Res 23:1933–1940
Morrow AL, VanDoren MJ, Penland SN, Matthews DB (2001) The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev 37:98–109
Morrow AL, Khisti RT, Tokunaga S, McDaniel JR, Matthews DB (2003) GABAergic neuroactive steroids modulate selective ethanol actions: mechanisms and significance. In: Smith SH (ed) Neurosteroid effects in the central nervous system: the role of the GABAA receptor. CRC Press, Miami, FL, pp 219–245
Nyberg S, Andersson A, Zingmark E, Wahlstrom G, Backstrom T, Sundstrom-Poromaa I (2005) The effect of a low dose of alcohol on allopregnanolone serum concentrations across the menstrual cycle in women with severe premenstrual syndrome and controls. Psychoneuroendocrinology 30:892–901
O'Dell LE, Alomary AA, Vallee M, Koob GF, Fitzgerald RL, Purdy RH (2004) Ethanol-induced increases in neuroactive steroids in the rat brain and plasma are absent in adrenalectomized and gonadectomized rats. Eur J Pharmacol 484:241–247
Paul SM, Purdy RH (1992) Neuroactive steroids. FASEB J 6:2311–2322
Penland S, Morrow AL (2004) 3α,5β-Reduced cortisol exhibits antagonist properties on cerebral cortical GABAA receptors. Eur J Pharmacol 506:129–132
Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Morrow AL, Kranzler HR (2005a) GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology 30:1193–1203
Pierucci-Lagha A, Covault J, Feinn R, Khisti RT, Morrow AL, Marx CE, Shampine LJ, Kranzler HR (2005b) Subjective effects and changes in steroid hormone concentrations in humans following acute consumption of alcohol. Psychopharmacology
Purdy RH, Morrow AL, Moore PH Jr, Paul SM (1991) Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A 88:4553–4557
Romeo E, Brancati A, De Lorenzo A, Fucci P, Furnari C, Pompili E, Sasso GF, Spalletta G, Troisi A, Pasini A (1996a) Marked decrease of plasma neuroactive steroids during alcohol withdrawal. J Pharmacol Exp Ther 247:309–322
Romeo E, Curatolo P, di Michele F, Spalletta G, Pompili E, Furnari C, Fucci P, Pasini A (1996b) Neurosteroid alterations in mood disorders associated with alcohol withdrawal. In: Genazzani AR, Petraglia F, Purdy RH (eds) The brain: source and target for sex steroid hormones. The Parthenon Group, New York, pp 113–122
Romeo E, Ströhle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R (1998) Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry 155:910–913
Sarkola T, Makisalo H, Fukunaga T, Eriksson CJ (1999) Acute effect of alcohol on estradiol, estrone, progesterone, prolactin, cortisol, and luteinizing hormone in premenopausal women. Alcohol Clin Exp Res 23:976–982
Seltzer CC (1971) Cigarettes and heart disease. N Engl J Med 284:557–558
Stern KN, McClintock MK (1996) Individual variation in biological rhythms: accurate measurement of preovulatory LH surge and menstrual cycle phase. In: Jensvold MF, Halbreich U, Hamilton JA (eds) Pyschopharmacology and women: sex, gender and hormones. American Psychiatric Press, Inc., Washington, DC, pp 11–42
Sutker PB, Goist KC Jr, King AR (1987) Acute alcohol intoxication in women: relationship to dose and menstrual cycle phase. Alcohol Clin Exp Res 11:74–79
Torres JM, Ortega E (2003) Alcohol intoxication increases allopregnanolone levels in female adolescent humans. Neuropsychopharmacology 28:1207–1209
Torres JM, Ortega E (2004) Alcohol intoxication increases allopregnanolone levels in male adolescent humans. Psychopharmacology 172:352–5
Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A (1998) Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A 95:3239–3244
Valimaki M, Harkonen M, Ylikahri R (1983) Acute effects of alcohol on female sex hormones. Alcohol Clin Exp Res 7:289–293
VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL (2000) Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci 20:1982–1989
Wechsler D (1958) The measurement and appraisal of adult intelligence. Williams & Wilkins, Baltimore, MD
Wiebe JP (1982) Identification of a unique sertoli cell steroid as 3α-hydroxy-4-pregnen-20-one (3α-dihydroprogesterone: 3α-DHP). Steroids 39:259–278
Xue BG, Whittemore ER, Park CH, Woodward RM, Lan NC, Gee KW (1997) Partial agonism by 3α,21-dihydroxy-5β-pregnan-20-one at the gamma-aminobutyric acid, receptor neurosteroid site. J Pharmacol Exp Ther 281:1095–1101
Acknowledgements
We thank Dr. Patrizia Porcu for critical comments and Lisa Vicini and Clare Tessman for assistance in preparation of the manuscript. This research was supported by DA02812 (HdW), MO1 RR00055 (University of Chicago General Clinical Research Center), and AA10564 (ALM). Preliminary results were presented at the 2002 Annual Meeting of the Research Society on Alcoholism.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holdstock, L., Penland, S.N., Morrow, A.L. et al. Moderate doses of ethanol fail to increase plasma levels of neurosteroid 3α-hydroxy-5α-pregnan-20-one-like immunoreactivity in healthy men and women. Psychopharmacology 186, 442–450 (2006). https://doi.org/10.1007/s00213-005-0187-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0187-0