Abstract
Rationale
Clinical reports indicate that acute exposure to 3,4-methylenedioxymethamphetamine (MDMA; “Ecstasy”) may induce pathological cerebrovascular responses in human users of the drug, however, the mechanism by which MDMA might effect these pathological changes is not clear.
Objectives
To examine the effects of acute MDMA administration on the relationship between local cerebral blood flow (LCBF) and local cerebral glucose utilisation (LCMRglu); to determine the effect, if any, acute exposure to MDMA has on the cerebral circulation, independently of alterations in cerebral metabolic demand.
Methods
Dark Agouti rats were injected with 15 mg.kg−1 i.p. MDMA or saline equivalent. LCBF and LCMRglu were measured in 50 brain areas using the fully quantitative [14C]iodoantipyrine and [14C]2-deoxyglucose autoradiographic techniques, respectively.
Results
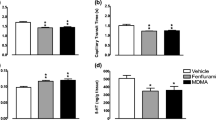
MDMA produced significant increases in LCMRglu in 23 brain areas, most markedly in the motor system (globus pallidus; +82%; medial striatum; +71%). In contrast, significant decreases in LCBF were observed in 28 brain areas, most markedly in primary sensory nuclei (superior colliculus; −32%) and limbic areas (anterior thalamus; −34%). Global analysis revealed a close correlation (r=0.87) between LCMRglu and LCBF with a ratio of 1.53 in controls. Despite the divergence of LCMRglu (increases) and LCBF (decreases) in MDMA-treated groups, there was a similar close correlation (r=0.84), but the ratio was decreased to 1.22.
Conclusions
This study provides clear evidence that acute exposure to MDMA results in cerebrovascular dysfunction. The uncoupling of LCBF from underlying metabolic demand, possibly due to the vasoconstrictor action of 5-HT, could provide the basis for oligaemia-induced pathological changes in the brain.






Similar content being viewed by others
References
Auer J, Berent R, Weber T, Lassnig E, Eber B (2002) Subarachnoid haemorrhage with “Ecstasy” abuse in a young adult. Neurol Sci 23:199–201
Battaglia G, Yeh SY, De Souza EB (1988) MDMA-induced neurotoxicity: parameters of degeneration and recovery of brain serotonin neurons. Pharmacol Biochem Behav 29:269–274
Colado MI, Williams JL, Green AR (1995) The hyperthermic and neurotoxic effects of ‘Ecstasy’ (MDMA) and 3,4 methylenedioxyamphetamine (MDA) in the Dark Agouti (DA) rat, a model of the CYP2D6 poor metabolizer phenotype. Br J Pharmacol 115:1281–1289
Cole J, Sumnall HR, Grob CS (2002) Sorted: ecstasy. Psychologist 15:464–467
Edvinsson L, MacKenzie ET (1977) Amine mechanisms in the cerebral circulation. Pharmacol Rev 28:275–348
Edvinsson L, Degueurce A, Duverger D, MacKenzie ET, Scatton B (1983) Central serotonergic nerves project to the pial vessels of the brain. Nature 306:55–57
Edvinsson L, McCulloch J, Sharkey J (1985) Vasomotor responses of cerebral arterioles in situ to putative dopaminergic receptor agonists. Br J Pharmacol 85:403–410
Frederick DL, Paule MG (1997) Effect of MDMA on complex brain function in laboratory animals. Neurosci Biobehav Rev 21:67–78
Green AR, Cross AJ, Goodwin GM (1995) Review of the pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA or ‘Ecstasy’). Psychopharmacology 119:247–260
Hanyu S, Ikeguchi K, Imai H, Imai N, Yoshida M (1995) Cerebral infarction associated with 3,4-methylenedioxymethamphetamine (‘ecstasy’) abuse. Eur Neurol 35:173
Harries DP, De Silva R (1992) ‘Ecstasy’ and intracerebral haemorrhage. Scott Med J 37:150–152
Kaku DA, Lowenstein DH (1990) Emergence of recreational drug abuse as a major risk factor for stroke in young adults. Ann Intern Med 113:821–827
Kelly PAT, McCulloch J (1984) Extrastriatal circuits activated by intrastriatal muscimol: a 14C-2-deoxyglucose investigation. Brain Res 292:357–366
Kelly PAT, Graham DI, Mcculloch J (1982) Specific alterations in local cerebral glucose utilization following striatal lesions. Brain Res 233:157–172
Kelly PAT, Ritchie IM, Collins FM (1995) Cerebrovascular consequences of repeated exposure to NG-nitro-L-arginine. Br J Pharmacol 116:2771–2777
Koch S, Galloway MP (1997) MDMA induced dopamine release in vivo: role of endogenous serotonin. J Neural Transm 104:135–146
Kokotos Leonardi ET, Azmitia EC (1994) MDMA (Ecstasy) inhibition of MAO type A and type B: comparisons with fenfluramine and fluoxetine (Prozac). Neuropsychopharmacology 10:231–238
Kuschinsky W, Wahl M (1978) Local chemical and neurogenic regulation of cerebrovascular resistance. Physiol Rev 58:656–689
Li AA, Marek GJ, Vocmer G, Seiden LS (1989) Long-term central 5-HT depletions resulting from repeated administration of MDMA enhances the effects of single administration of schedule-controlled behaviour of rats. Pharmacol Biochem Behav 33:641–648
Lou HC, Edvinsson L, MacKenzie ET (1987) The concept of coupling blood flow to brain function: revision required? Ann Neurol 22:289–297
Malpass A, White JM, Irvine RJ, Somogyi AA, Bochner F (1999) Acute toxicity of 3,4-methylenedioxymethamphetamine (MDMA) in Sprague-Dawley and Dark Agouti rats. Pharmacol Biochem Behav 64:29–34
McBean DE, Sharkey J, Ritchie IM, Kelly PAT (1990) Evidence for a possible role for serotonergic systems in the control of cerebral blood flow. Brain Res 537:307–310
McBean DE, Sharkey J, Ritchie IM, Kelly PAT (1991) Cerebrovascular and functional consequences of 5-HT1A receptor activation. Brain Res 555:159–163
McBean DE, Ritchie IM, Olverman HJ, Kelly PAT(1999) Effects of the specific serotonin reuptake inhibitor, citalopram, upon local cerebral blood flow and glucose utilisation in the rat. Brain Res 847:80–84
McCulloch J, Kelly PAT, Ford I (1982) The effect of apomorphine on the relationship between local cerebral glucose utilization and local cerebral blood flow with an appendix on its statistical analysis. J Cereb Blood Flow Metab 2:487–499
McEvoy AE, Kitchen ND, Thomas DGT (2000) Intracerebral haemorrhage in young adults: the emerging importance of drug misuse. BMJ 320:1322–1324
McKenna DJ, Peroutka SJ (1990) Neurochemistry and neurotoxicity of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”). J Neurochem 54:14–22
Miranda J, O’Neill D (2002) Stroke associated with amphetamine use. Ir Med J 95:281–282
Nichols DE (1986) Differences between the mechanism of action of MDMA, MBDB and the classic hallucinogens. Identification of a new therapeutic class: entactogens. J Psychoactive Drugs 18:305–313
Nichols DE, Oberlender R (1990) Structure-activity relationships of MDMA and related compounds: a new class of psychoactive drugs? Ann N Y Acad Sci 600:612–623
O’Loinsigh ED, Boland G, Kelly JP, O’Boyle KM (2001) Behavioural, hyperthermic and neurotoxic effects of 3,4-methylenedioxymethamphetamine analogues in the Wistar rat. Prog Neuropsychopharmacol Biol Psychiatry 25:621–638
O’Shea E, Granados R, Esteban B, Colado MI, Green AR (1998) The relationship between the degree of neurodegeneration of rat brain 5-HT nerve terminals and the dose and frequency of administration of MDMA (‘ecstasy’). Neuropharmacology 37:919–926
Parrot AC (2001) Human psychopharmacology of ecstasy (MDMA): a review of 15 years of empirical research. Hum Psychopharmacol 16:557–577
Perez JA, Arsura EL, Strategos S (1999) Methamphetamine-related stroke: four cases. J Emerg Med 17:469–471
Petitti DB, Sidney S, Queensberry C, Bernstein A (1998) Stroke and cocaine or amphetamine use. Epidemiology 9:596–600
Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS (2001) Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39:32–41
Rudnick G, Wall SC (1992) The molecular mechanism of “ecstasy” [3,4-methylenedioxymethamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci U S A 89:1817–1821
Sakurada O, Kennedy C, Jehle J, Brown JD, Carbin GL, Sokoloff L (1978) Measurement of local cerebral blood flow with iodo[14C]antipyrine. Am J Physiol 234:H59–H66
Scanzello CR, Hatzidimitriou G, Martello AL, Katz JL, Ricaurte GA (1993) Serotonergic recovery after (±)3,4-(methylenedioxy)methamphetamine injury: observations in rats. J Pharmacol Exp Ther 264:1484–1491
Schmidt CJ (1987) Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J Pharmacol Exp Ther 240:1–7
Schmidt CJ (1989) Acute and long-term neurochemical effects of methylenedioxymethamphetamine in the rat. NIDA Res Monogr 94:179–195
Schmidt CJ, Wu L, Lovenberg W (1986) Methylenedioxymethamphetamine: a potentially neurotoxic amphetamine analogue. Eur J Pharmacol 124:175–178
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M (1977) The [14C]deoxyglucose method for the measurement of local cerebral glucose utilisation: theory, procedure and normal values in the conscious and anesthetized albino rat. J Neurochem 28:897–916
Stone DM, Stahl DC, Hanson GR, Gibb JW (1986) The effects of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) on monoaminergic systems in the rat brain. Eur J Pharmacol 128:41–48
Stone DM, Merchant KM, Hanson GR, Gibb JW (1987) Immediate and long-term effects of 3,4-methylenedioxymethamphetamine on serotonin pathways in brain of rat. Neuropharmacology 26:1677–1683
Tucker GT, Lennard MS, Ellis SW, Woods HF, Cho AK, Lin LY, Hiratsuka A, Schmitz DA, Chu TYY (1994) The demethylenation of methylenedioxymethamphetamine (“Ecstasy”) by debrisoquine hydroxylase (CYP2D6). Biochem Pharmacol 47:1151–1156
White SR, Obradovic T, Imel KM, Wheaton MJ (1996) The effects of methylenedioxymethamphetamine (MDMA, ‘Ecstasy’) on monoaminergic neurotransmission in the central nervous system. Prog Neurobiol 49:455–479
Wichems CH, Hollingsworth CK, Bennett BA (1995) Release of serotonin induced by 3,4-methylenedioxymethamphetamine (MDMA) and other substituted amphetamines in cultured fetal raphe neurons: further evidence for calcium-independent mechanisms of release. Brain Res 695:10–18
Wilkerson G, London ED (1989) Effects of methylenedioxymethamphetamine on local cerebral glucose utilization in the rat. Neuropharmacology 28:1129–1138
Acknowledgements
This study was funded by EC grant no. QLG3-CT-2002–00809, and by the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quate, L., McBean, D.E., Ritchie, I.M. et al. Acute methylenedioxymethamphetamine administration: effects on local cerebral blood flow and glucose utilisation in the dark agouti rat. Psychopharmacology 173, 287–295 (2004). https://doi.org/10.1007/s00213-004-1784-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-004-1784-z