Abstract
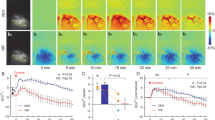
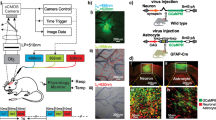
Cocaine-induced vasoconstriction reduces blood flow, which can jeopardize neuronal function and in the prefrontal cortex (PFC) it may contribute to compulsive cocaine intake. Here, we used integrated optical imaging in a rat self-administration and a mouse noncontingent model, to investigate whether changes in the cerebrovascular system in the PFC contribute to cocaine self-administration, and whether they recover with detoxification. In both animal models, cocaine induced severe vasoconstriction and marked reductions in cerebral blood flow (CBF) in the PFC, which were exacerbated with chronic exposure and with escalation of cocaine intake. Though there was a significant proliferation of blood vessels in areas of vasoconstriction (angiogenesis), CBF remained reduced even after 1 month of detoxification. Treatment with Nifedipine (Ca2+ antagonist and vasodilator) prevented cocaine-induced CBF decreases and neuronal Ca2+ changes in the PFC, and decreased cocaine intake and blocked reinstatement of drug seeking. These findings provide support for the hypothesis that cocaine-induced CBF reductions lead to neuronal deficits that contribute to hypofrontality and to compulsive-like cocaine intake in addiction, and document that these deficits persist at least one month after detoxification. Our preliminary data showed that nifedipine might be beneficial in preventing cocaine-induced vascular toxicity and in reducing cocaine intake and preventing relapse.






Similar content being viewed by others
References
Rapinesi C, et al. Add-on high frequency deep transcranial magnetic stimulation (dTMS) to bilateral prefrontal cortex reduces cocaine craving in patients with cocaine use disorder. Neurosci Lett. 2016 Aug 26;629:43–47.
Volkow ND, Morales M, The Brain on Drugs: From Reward to Addiction, Cell. 2015 Aug 13; 162(4):712–25.
Levine SR, et al. “Crack” cocaine-associated stroke. Neurology. 1987;37:1849–53.
Levine SR, et al. A comparative study of the cerebrovascular complications of cocaine: alkaloidal versus hydrochloride—a review. Neurology. 1991;41:1173–7.
Tuchman AJ, Daras M, Zalzal P, Mangiardi J. Intracranial hemorrhage after cocaine abuse. J Am Med Assoc. 1987;257:1175.
Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. Cerebral blood flow in chronic-cocaine users: a study with positron emission tomography. Br J Psychiatry. 1988;152:641–8.
Zhang Q, et al. Chronic-cocaine disrupts neurovascular networks and cerebral function: optical imaging studies in rodents. J Biomed Opt. 2016;21:26006.
Ren H, et al. Cocaine-induced cortical microischemia in the rodent brain: clinical implications. Mol Psychiatry. 2012;17:1017–25.
You J, Du C, Volkow ND, Pan Y. Optical coherence Doppler tomography for quantitative cerebral blood flow imaging. Biomed Opt Express. 2014;5:3217–30.
Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300.
Koob GF, in Addiction Medicine: Science and Practice, AB Johnson, editor. New York, NY: Springer; 2011. p. 333–57.
Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl. 1):S18–S31.
Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology. 2009;205:565–75.
Yuan Z, Luo Z, Volkow ND, Pan Y, Du C. Imaging separation of neuronal from vascular effects of cocaine on rat cortical brain in vivo. Neuroimage. 2011;54:1130–9.
Araki H, Hino N, Karasawa Y, Kawasaki H, Gomita Y. Effect of calcium channel blockers on cerebral ischemia-induced hyperactivity in Mongolian gerbils. Physiol Behav. 1999;67:573–7.
Chen W, Liu P, Volkow ND, Pan Y, Du C. Cocaine attenuates blood flow but not neuronal responses to stimulation while preserving neurovascular coupling for resting brain activity. Mol Psychiatry. 2016;21:1408–16.
Gu X, Chen W, You J, Koretsky AP, Volkow ND, Pan Y, et al. Long-term optical imaging of neurovascular coupling in mouse cortex using GCaMP6f and intrinsic hemodynamic signals. Neuroimage. 2018;165:251–64.
Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Elsevier Academic Press; 2004.
You J, Zhang Q, Park K, Du C, Pan Y. Quantitative imaging of microvascular blood flow networks in deep cortical layers by 1310 nm μODT. Opt Lett. 2015;40:4293–6.
Zhao Y, et al. Phase-resolved optical coherence tomography and optical Doppler tomography for imaging blood flow in human skin with fast scanning speed and high velocity sensitivity. Opt Lett. 2000;25:114–6.
Palagyi K, Kuba A. A 3D 6-subiteration thinning algorithm for extracting medial lines. Pattern Recognition Letters, 1998;19:627.
McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug seeking behavior. J Neurosci. 2001;21:8655–63.
Park K, You J, Du C, Pan Y. Cranial window implantation on mouse cortex to study microvascular change induced by cocaine. Quant Imaging Med Surg. 2015;5:97–107.
He GQ, Zhang A, Altura BT, Altura BM. Cocaine-induced cerebrovasospasm and its possible mechanism of action. J Pharmacol Exp Ther. 1994;268:1532.
Shen Y, Pu IM, Ahearn T, Clemence M, Schwarzbauer C. Quantification of venous vessel size in human brain in response to hypercapnia and hyperoxia using magnetic resonance imaging. Magn Reson Med. 2013;69:1541–52.
Abbound FM, Eckstein JW, Zimmerman BG, Graham MH. Sensitization of arteries, veins, and small vessels to norepinephrine after cocaine. Circ Res. 1964;15:247–57.
Sofuoglu M, Nelson D, Babb DA, Hatsukami DK. Intravenous cocaine increases plasma epinephrine and norepinephrine in humans. Pharmacol Biochem Behav. 2001;68:455–9.
Kalsner S. Cocaine sensitization of coronary artery contractions: mechanism of drug-induced spasm. J Pharmacol Exp Ther. 1993;264:1132–40.
Laporte R, DeRoth L. Modulation of the effects of norepinephrine uptake inhibitors on the norepinephrine-induced contractile response of the porcine uterine artery during early pregnancy. Can J Vet Res. 1997;61:214–20.
Ohtsuki S, Yamaguchi H, Kang YS, Hori S, Terasaki T. Reduction of L-type amino acid transporter 1 mRNA expression in brain capillaries in a mouse model of Parkinson’s disease. Biol Pharm Bull. 2010;33:1250–2.
Krimer LS, Muly EC, Williams GV, Goldman-Rakic PS. Dopaminergic regulation of cerebral cortical microcirculation. Nat Neurosci. 1998;1:286–9.
Giessler C, Wangemann T, Silber RE, Dhein S, Brodde OE. Noradrenaline-induced contraction of human saphenous vein and human internal mammary artery: involvement of different alpha-adrenoceptor subtypes. Naunyn Schmiede Arch Pharmacol. 2002;366:104–9. Epub 2002 Jun 14
Chen W, Volkow ND, Li J, Pan Y, Du C. Cocaine decreases spontaneous neuronal activity and increases low-frequency neuronal and hemodynamic cortical oscillations, Cereb Cortex. 2018. https://doi.org/10.1093/cercor/bhy057
Al-Rawi PG, Kirkpatrick PJ. Tissue oxygen index: thresholds for cerebral ischemia using near-infrared spectroscopy. Strokec. 2006;37:2720–5.
Sordo L, et al. Cocaine use and risk of stroke: a systematic review. Drug Alcohol Depend. 2014;142:1–13.
Toossi S, Hess CP, Hills NK, Josephson SA. Neurovascular complications of cocaine use at a tertiary stroke center. J Stroke Cerebrovasc Dis. 2010;19:273–8.
Treadwell SD, Robinson TG. Cocaine use and stroke. Postgrad Med J. 2007;83:389–94.
You J, Volkow ND, Park K, Zhang Q, Clare K, Du C, et al. Cerebrovascular adaptations to cocaine-induced transient ischemic attacks in the rodent brain. JCI Insight. 2017;2:e90809 https://doi.org/10.1172/jci.insight.90809
Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–80.
Strong DH, et al. Eosinophilic “empyema” associated with crack cocaine use. Thorax. 2003;58:823–4.
Marti HJ, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–76.
Zhang ZG, et al. Correlation of VEGF and angiopoietin expression with disruption of blood–brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–92.
Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–8.
Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–84.
George O, Mandyam CD, Wee S, Koob GF. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology. 2008;33:2474–82. Epub 2007 Nov 21
Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–62.
Cunningham JJ, Orr E, Lothian BC, Morgen J, Brebner K Effects of fendiline on cocaine-seeking behavior in the rat. Psychopharmacology (Berl). 2015;232:4401–10.
Muntaner C, Kumor KM, Nagoshi C, Jaffe JH. Effects of nifedipine pretreatment on subjective and cardiovascular responses to intravenous cocaine in humans. Psychopharmacology (Berl). 1991;105:37–41.
Calcagnetti DJ, Keck BJ, Quatrella LA, Schechter MD Blockade of cocaine-induced conditioned place preference: relevance to cocaine abuse therapeutics. Life Sci. 1995;56:475–83.
Degoulet M, Stelly CE, Ahn KC, Morikawa H. L-type Ca2+ channel blockade with antihypertensive medication disrupts VTA synaptic plasticity and drug-associated contextual memory. Mol Psychiatry. 2016;21:394–402.
Buttner A. Review: the neuropathology of drug abuse. Neuropathol Appl Neurobiol. 2011;37:118–34.
Volkow ND, Morales M. The brain on drugs: from reward to addiction. Cell . 2015;162:712–25.
Maekawa T, Tommasino C, Shapiro HM, Keifer-Goodman J, Kohlenberger RW. Local cerebral blood flow and glucose utilization during isoflurane anesthesia in the rat. Anesthesiology. 1986;65:144–51.
Edvinsson L, Johansson BB, Larsson B, MacKenzie ET, Skarby T, Young AR. Calcium antagonists: effects on cerebral blood flow and blood–brain barrier permeability in the rat. Br J Pharmacol. 1983;79:141–8.
Acknowledgments
We specially thank to Sunmee Wee for conducting the initial self-administration experiment and analyzing that behavior data. Also to J. Li for partially assisting with figure illustration (Fig. 5) and Q.J. Zhang for assisting with noncontingent cocaine administration of rats and their VEGF studies on PFC and the somatosensory cortex. This work was supported in part by National Institutes of Health (NIH) grants 1R01DA029718 (C.D. and Y.P.), R21DA042597 (Y.P. and C.D.), R01DA04398 (G.F.K. when he was at The Scripps Research Institute), and NIH’s Intramural Program of NIAAA (NDV). The authors would also like to thank the NIDA drug supply program for providing the cocaine used in the calcium antagonist and mouse model experiments.
Author contributions
C.D. and Y.P. designed and built the optical setups. N.D.V., C.D., and Y.P. designed the experiments. G.F.K. designed the self-administration model and provided self-administering animals and helped with analysis and interpretation of the results. C.P.A. conducted self-administration animals for Ca2+ antagonist studies. C.P.A., J.Y., and K.P. carried out the imaging experiments and data analysis. All authors proofread the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Du, C., Volkow, N.D., You, J. et al. Cocaine-induced ischemia in prefrontal cortex is associated with escalation of cocaine intake in rodents. Mol Psychiatry 25, 1759–1776 (2020). https://doi.org/10.1038/s41380-018-0261-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-018-0261-8
- Springer Nature Limited
This article is cited by
-
Astrocytes modulate cerebral blood flow and neuronal response to cocaine in prefrontal cortex
Molecular Psychiatry (2024)
-
Dynamic 3D imaging of cerebral blood flow in awake mice using self-supervised-learning-enhanced optical coherence Doppler tomography
Communications Biology (2023)
-
Unveiling OASIS family as a key player in hypoxia–ischemia cases induced by cocaine using generative adversarial networks
Scientific Reports (2022)
-
Cocaine’s cerebrovascular vasoconstriction is associated with astrocytic Ca2+ increase in mice
Communications Biology (2022)
-
Ca2+ channel blockade reduces cocaine’s vasoconstriction and neurotoxicity in the prefrontal cortex
Translational Psychiatry (2021)