Abstract.
We examined the contribution of K+ channels to the relaxation responses induced by different redox forms of nitric oxide (NO•, NO– and NO+) in comparison with those evoked by electrical field stimulation (EFS) of nitrergic nerves in the sheep urethra. K+ channel blockers with different selectivity profile were used. Sodium nitroprusside (SNP) and different S-nitrosothiols were used as NO+ donors, Angeli's salt as an NO– donor and nitroglycerin (GTN) was chosen as a representative compound known to require metabolic activation in the target tissue. Pure NO gas was used to prepare NO• solutions.
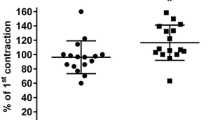
Relaxation evoked by EFS of nitrergic nerves or by exogenous NO• was not inhibited by any of the K+ channel blockers, but was enhanced by 4-aminopyridine [inhibitor of voltage-dependent K+ (Kv) channels]. This suggests that, whereas K+ channel activation and hyperpolarization of the postsynaptic membrane do not contribute to relaxation, prejunctional modulation of the nitrergic neurotransmission by Kv channels may be relevant. Relaxation induced by NO+ or NO– donors was not affected by K+ channel blockade with the following exceptions: glybenclamide, a blocker of ATP-sensitive K+ channels (KATP), enhanced responses to SNP and Angeli's salt, 4-aminopyridine inhibited relaxation evoked by Angeli's salt and GTN, and charybdotoxin, a blocker of large-conductance, Ca2+-activated K+ channels (BKCa) inhibited those induced by the S-nitrosothiol S-nitrosoglutathione. These results do not suggest the existence of a general mechanism of action on K+ channels for compounds releasing either NO+ or NO– in the sheep urethra. None of the K+ channel blockers affected relaxation induced by the membrane-permeable analogue of cGMP, 8-bromo-cGMP. However, the fact that the addition of the phosphodiesterase inhibitor zaprinast (0.1 mM) enhanced the relaxation to Angeli's salt, while preventing the inhibition induced by 4-aminopyridine, suggests that involvement of guanylate cyclase activation in the action of NO donors on K+ channels can not be excluded. Accordingly, the guanylate cyclase inhibitors 1H-[1,2,4]-oxadiazole-[4,3-a]-quinoxalin-1-one (ODQ, 10 µM) and 4H-8-bromo-1,2,4-oxadiazolo(3,4-d)benz(b)(1,4)oxazin-1-one (NS 2028, 10 µM) almost abolished relaxations to EFS and Angeli's salt. In contrast, ODQ only moderately inhibited relaxations to NO•. In addition, the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethyl imidazoline-1-oxyl 3-oxide (carboxy-PTIO) effectively inhibited responses to NO• whilst not affecting those to EFS or NO–, suggesting a close similarity between the nitrergic transmitter and nitroxyl ion.
We conclude that nitrergic relaxation induced either by the endogenous transmitter or by exogenous NO donors in the ovine urethra is not mediated by postsynaptic alterations in the K+ conductance; only a prejunctional modulation through Kv channels seems to be significant. In addition, the production and/or release of alternative redox forms of NO, such as NO–, may be involved in neurotransmission processes in the urethra.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Costa, G., Labadía, A., Triguero, D. et al. Nitrergic relaxation in urethral smooth muscle: involvement of potassium channels and alternative redox forms of NO. Naunyn-Schmied Arch Pharmacol 364, 516–523 (2001). https://doi.org/10.1007/s002100100480
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s002100100480