Abstract
Methotrexate (MTX) is a folic acid reductase inhibitor that manages various malignancies as well as immune-mediated inflammatory chronic diseases. Despite being frequently prescribed, MTX’s severe multiple toxicities can occasionally limit its therapeutic potential. Intestinal toxicity is a severe adverse effect associated with the administration of MTX, and patients are significantly burdened by MTX-provoked intestinal mucositis. However, the mechanism of such intestinal toxicity is not entirely understood, mechanistic studies demonstrated oxidative stress and inflammatory reactions as key factors that lead to the development of MTX-induced intestinal injury. Besides, MTX causes intestinal cells to express pro-inflammatory cytokines like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), which activate nuclear factor-kappa B (NF-κB). This is followed by the activation of the Janus kinase/signal transducer and activator of the transcription3 (JAK/STAT3) signaling pathway. Moreover, because of its dual anti-inflammatory and antioxidative properties, nuclear factor erythroid-2-related factor 2/heme oxygenase-1 (Nrf2/HO-1) has been considered a critical signaling pathway that counteracts oxidative stress in MTX-induced intestinal injury. Several agents have potential protective effects in counteracting MTX-provoked intestinal injury such as omega-3 polyunsaturated fatty acids, taurine, umbelliferone, vinpocetine, perindopril, rutin, hesperidin, lycopene, quercetin, apocynin, lactobacillus, berberine, zinc, and nifuroxazide. This review aims to summarize the potential redox molecular mechanisms of MTX-induced intestinal injury and how they can be alleviated. In conclusion, studying these molecular pathways might open the way for early alleviation of the intestinal damage and the development of various agent plans to attenuate MTX-mediated intestinal injury.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intestinal mucosal injury is a common unfavorable side effect of chemotherapy, which arises from the drugs’ inability to distinguish between normal and tumor cells. The intestinal epithelial cells are frequently attacked during chemotherapy treatments due to their ability to proliferate quickly (Dahlgren et al. 2021). Up to 25–75% of cancer patients undergoing various chemotherapy treatments experience chemotherapy-induced intestinal mucosal injury which can include diarrhea, a decline in quality of life, treatment intolerance that forces discontinuation of drugs, and even mortality (Li et al. 2023). Methotrexate (MTX), a chemotherapeutic medication that has a folate antagonistic effect, is frequently used to treat multiple types of cancers, including breast cancer and lymphoma as well as immune-mediated inflammatory chronic diseases (Joerger et al. 2012). Unfortunately, MTX cytotoxicity is not limited to cancer cells but extends to affect non-cancer cells of vital organs such as intestinal mucosa (Tang et al. 2020). Gastrointestinal toxicity by MTX causes nausea, vomiting, and loss of nutrient absorption. Enteritis is distinguished histologically by crypt loss and atrophy of intestinal villi (Miyazono et al. 2004). Administration of MTX impairs mucosa barrier function, which causes bacterial translocation and inflammation. Also, its administration results in intestinal damage involving notable morphological small intestine injury and mucosal damage (Huang et al. 2020). Besides, MTX treatment causes DNA strands to break in intestinal epithelial cells that proliferate quickly (Sonis 2004) and induces oxidative stress (El-Sheikh et al. 2016; Gautam et al. 2016). More significantly, MTX may have harmful consequences by inducing a dynamic series of inflammatory events in the intestinal epithelium and submucosal tissues that are initiated by direct cellular damage (Sonis 2004; Sonis et al. 2004). Consequently, the purpose of the underlying review is to elucidate the potential molecular mechanisms of MTX-induced intestinal injury and study the protective strategies involved in the amelioration of this injury. In particular, we aimed to assess the role of inflammation and oxidative stress with a focus on the nuclear factor-kappa B (NF-κB), the Janus kinase/signal transducer and activator of the transcription3 (JAK/STAT3), nuclear factor erythroid-2-related factor 2/heme oxygenase-1 (Nrf2/HO-1), peroxisome proliferator-activated receptor-gamma (PPAR-γ), and silent information regulator-1 (SIRT1) in pathogenesis of intestinal injury induced by MTX. A deeper understanding of the molecular mechanisms involved in MTX-induced intestinal injury may help to explain a number of the drug’s toxicities and develop multiple strategies to be investigated to ameliorate the harmful adverse effects of MTX.
Chemical properties of methotrexate
The antifolate medication MTX, also referred to 4-amino-N10-methylpteroylglutamic acid, was developed in 1940 as the first anticancer medication (Abolmaali et al. 2013). MTX and folic acid have remarkably similar structures. The structure of MTX consists of a pteridine-diamine core and a p-amino benzoyl portion connected to a glutamic acid segment containing two highly ionizable carboxylic acid groups. Since MTX must dissolve in neutral or basic solutions, its solubility is pH-dependent. S and R stereoisomers are the product of an asymmetric carbon in the molecule. The R isomer is considered an impurity, and S-MTX is regarded as the active form (Guichard et al. 2017). It is evident that the substitution of an amino function for the hydroxyl group on C2 and the methylation of N10 are the primary structural differences between MTX and the structure of naturally occurring folic acid (Rubino 2001). The structures of folic acid, MTX, and its three main metabolites are illustrated in Fig. 1.
Indication of methotrexate
MTX is an FDA-approved drug for treating rheumatoid arthritis patients. It may also be useful in patients suffering from juvenile idiopathic arthritis (Braun 2010). MTX was used initially in rheumatoid arthritis after a double-blind, placebo-controlled clinical trial of MTX in rheumatoid arthritis patients (Weinblatt et al. 1985). Nowadays, MTX is one of the main chemotherapeutic options for treating different kinds of cancer. It is frequently used to manage several cancer types including lung cancer, lymphoma, bladder cancer, and breast cancer (Joerger et al. 2012; Khan et al. 2012) and at low doses for many autoimmune illnesses like systemic lupus erythematosus (Cipriani et al. 2014; Bedoui et al. 2019). Besides, European and American guidelines recommend the use of MTX for active Crohn’s disease and psoriasis (Gomollón et al. 2017; Coates et al. 2020; Nielsen et al. 2020). Additionally, MTX has demonstrated efficacy when paired with anti-tumor necrosis factor-alpha (TNF-α) drugs in the treatment of individuals suffering from ulcerative colitis, breast cancer, lung carcinoma, head and neck malignancies, and ovarian carcinoma (Chande et al. 2014).
Mechanism of action of methotrexate in cancer and autoimmune diseases
MTX has a special mode of action when used in chemotherapy and immunosuppression in autoimmune conditions. In cancer, MTX acts as an antifolate antimetabolite. When MTX enters a cell through carriers referred to as human-reduced folate carriers (SLC19A1), polyglutamate synthetase attaches glutamate residues to the γ-carboxylate group of MTX, converting it into methotrexate polyglutamates (MTX-PGs). Glutamyl hydrolase, on the other hand, converts MTX-PGs back into MTX. Dihydrofolate reductase (DHFR) which catalyzes the conversion of dihydrofolate into tetrahydrofolate, the active form of folic acid, is competitively inhibited by MTX and MTX-PGs, which depletes vital tetrahydrofolate (THF) needed for cellular functions (Xu et al. 2022). DNA and RNA synthesis is inhibited by the dual inhibitory action of MTX-PGs on thymine synthase and DHFR, which results in the inhibition of purine and pyrimidine de novo synthesis (Giletti and Esperon 2018; Cheng et al. 2021; Mikhaylov et al. 2019). DHFR is a key enzyme in the process of thymidylate synthesis, catalyzing the folate reduction to THF in two steps: during the first step, folates are reduced to dihydrofolate (DHF), which are further reduced to THF (Neradil et al. 2012). The organism contains several active folate forms, including 5-methyl-THF, 10-formyl-THF, and 5,10-methylene-THF, which are donors of monocarbon units like methyl, formyl, and methylene (Assaraf 2006). The hydroxy methyltransferase enzyme, which is also involved in the conversion of L-serine to glycine, mediates the conversion of THF to 5,10-methylene-THF. 5,10-Methylene-THF is a carbon donor and coenzyme in the methylation of 2-deoxyuridine-5-monophosphate (dUMP) to 2-deoxythymidine-5-monophosphate (dTMP) which is being mediated by thymidylate synthase. Lack of THF directly affects de novo pyrimidine synthesis (Rao et al. 2003). THF is necessary for the synthesis of the nucleotides in both DNA and RNA. MTX-polyglutamate further suppresses DNA synthesis by blocking the de novo production of purine and thymidylate synthase (Singh et al. 2019). For autoimmune diseases, MTX is the recommended medication for several reasons. MTX-glu inhibits the folate pathway component thymidylate synthetase that promotes thymine nucleoside residue generation. Additionally, MTX-glu prevents the conversion of 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) to formaminoimidazole-4-carboxamide ribonucleotide (FAICAR) by inhibiting the key enzyme AICAR transformylase (ATIC) in the purines de novo synthesis pathway, leading to the accumulation of AICAR (Friedman and Cronstein 2019). The release of adenosine into extracellular space is promoted by the buildup of AICAR. Adenosine has an anti-inflammatory effect by interacting with receptors on neutrophils and monocytes (Whittle and Hughes 2004). Because of its anti-inflammatory properties, adenosine inhibits methyltransferase activity, which stops interleukin-1beta (IL-1β) from binding to its cell surface receptor, suppresses T-cell activation, down-regulates B-cells, and enhances the sensitivity of activated CD-95 T cells (Mikhaylov et al. 2019; Tukukino and Wallerstedt 2020). By blocking ATIC with MTX-glu, pro-inflammatory cytokines such as TNF-α, IL-1, and IL-6 can also be significantly decreased (Budzik et al. 2000).
Administration of methotrexate
MTX is usually given as a single weekly dose in treating autoimmune disorders. In clinical practice, based on clinical response or intolerance, the starting dose of medication is 10 mg/week, with increases of 5 mg every 2–4 weeks, up to a maximum dose of 20–30 mg/week (Visser and van der Heijde 2009; Inoue and Yuasa 2014). MTX monotherapy has been included in recent clinical guidelines for the treatment and remission maintenance of active Crohn’s disease (Torres et al. 2020; Feuerstein et al. 2021). Previous publications demonstrated the effectiveness of intramuscular (IM) MTX at a dose of 25 mg/week for 12 months in a randomized controlled trial including thiopurine-naïve patients with Crohn’s disease (Feagan et al. 1995; Park et al. 2023a). The use of parenteral MTX has gained popularity recently and is more beneficial than taking it orally, especially when administered subcutaneously (SC). It has been demonstrated that SC MTX is more clinically effective and has better tolerance than the oral route. Currently, when oral MTX is not tolerated or shows insufficient clinical response, SC MTX treatment is advised (Visser and van der Heijde 2009; Bello et al. 2017).
Pharmacokinetic of methotrexate
Absorption
Following oral treatment, MTX is absorbed in the proximal jejunum by the proton-coupled folate transporter (PCFT/SLC46A1), which then transports reduced folates in addition to MTX (Desmoulin et al. 2012). A tiny amount of MTX may be converted by intestinal bacteria to 4-amino-4-deoxy-N10-methylpterroic acid (Grim et al. 2003). Although MTX has a relatively high bioavailability (64–90%), it varies significantly among patients and reaches a plateau at doses beyond 15 mg/week, suggesting intestinal transporter saturation (Hillson and Furst 1997; Hoekstra et al. 2004; Schiff et al. 2014). Studies have shown that SC MTX has a better bioavailability than oral MTX (Hoekstra et al. 2004; Bianchi et al. 2016).
MTX bioavailability varies from 30 to 90% in different people. Under fasting conditions, MTX’s Tmax was attained in 0.75–2 h, and its Cmax ranged from 0.3 to 1.6 µmol/L. Food does not considerably alter the bioavailability of MTX; however, it slightly prolongs the Tmax and decreases the Cmax. Various administration routes result in varying medication concentrations. When MTX is injected with IM or SC, the serum concentration is extremely high (Rajitha et al. 2017). The concentration of MTX in the synovium is significantly higher than that in the serum following intraarticular injection. The synovial MTX concentration is equivalent to the plasma concentration after oral or IM treatment (Rajitha et al. 2017).
The oral absorption of MTX is rapid but incomplete due to factors like receptor saturation, the inhibitory effect of food on its absorption, and rapid metabolization by gut flora (Attwa et al. 2019). The SC method of administration of MTX is becoming more popular, despite the oral route still being used the most commonly. The reason for this is that multiple trials have shown that the bioavailability of SC MTX is greater, and the bioavailability of oral MTX is somewhat variable. The bioavailability of oral MTX exhibits significant interpatient variability and a plateaued effect at doses over 15 mg/week. In contrast, the bioavailability of SC MTX is dose-dependent and linear, displaying no plateau (Schiff et al. 2014). In fact, some clinical trials have also suggested switching from the oral to the parenteral route of MTX administration (Jundt et al. 1993; Hoque et al. 2023). Studies on SC MTX have also revealed a good risk-benefit profile, indicating that the SC route may be superior to the oral route for administering higher doses of MTX because of the speed and sustainability of response (Warren et al. 2017; Dogra et al. 2022).
It is currently unclear what mechanism underlying the saturation and variability of oral bioavailability of MTX in clinical practice (Murakami and Mori 2012). Besides, the increase in oral bioavailability by administration of a higher oral dose of MTX suggests the contribution of saturated transport in oral bioavailability (VanWert and Sweet 2008). MTX is recognized as a substrate for several transporters, such as solute carrier (SLC) influx transporters and ATP-binding cassette transporters (ABC) efflux transporters. Regarding SLC transporters, reduced folate carrier (RFC), proton-coupled folate transporter (PCFT), organic anion transporter 3 (OAT3), and organic anion transporting polypeptide 1A2 (OATP1A2) all transport MTX as a substrate (Badagnani et al. 2006; Shibayama et al. 2006). Intestines exhibit PCFT and RFC, with PCFT being particularly expressed in the brush-border membranes of the proximal small intestine (Urquhart et al. 2010). Multiple strategies for optimizing MTX dosing regimens should be followed to ensure consistent drug exposure in patients. Oral MTX responsiveness can be enhanced by administering a large starting dose and rapidly titrating the medication; this approach does not seem to compromise safety or tolerability (Bello et al. 2017). Patients who are not able to tolerate MTX treatment or whose efficacy is insufficient can be “rescued” by converting to SC MTX. Beginning with SC MTX should also be taken into consideration due to its advantageous pharmacokinetic profile and absorption (Tornero Molina et al. 2021). Treatment persistence is probably going to be improved if patients are started on SC MTX or switch from oral to SC delivery (Li et al. 2021a).
Distribution
MTX can be distributed to synovial fluid in amounts similar to those in plasma (Herman et al. 1989).
Metabolism
One of MTX’s main metabolites, 7-hydroxymethotrexate (7-OH-MTX), is produced by the liver during the first-pass metabolism of MTX (Seideman et al. 1993).
Excretion
Renal excretion is the main route of MTX elimination. The medication goes through active tubular secretion and reabsorption in addition to being filtered by the glomeruli. Bile excretes a tiny amount of MTX, and some enterohepatic recycling also takes place (Nuernberg et al. 1990; Seideman et al. 1993).
Renal elimination is the primary route of excretion for both MTX and its metabolites. This process involves glomerular filtration, tubular secretion, and tubular reabsorption. Tubular secretion and reabsorption have high interindividual variability, and both can be saturated which can result in nonlinear pharmacokinetics (Van Roon and Van De Laar 2010; Maksimovic et al. 2020). Between 2 and 12% of patients receiving high-dose MTX therapy may get acute kidney injury, mostly as a result of crystal nephropathy caused by MTX and its metabolite, 7-OH-MTX. Under acidic conditions, MTX and its metabolite, 7-OH-MTX, precipitate pH-dependent crystals within the tubular lumen of renal tubules. Urine alkalinization dramatically improves MTX and 7-OH-MTX solubility and excretion reducing medication toxicities (Howard et al. 2016; Reed et al. 2019). Since more than 90% of MTX is excreted by the renal tubules, any kidney problem could result in inefficient elimination of MTX. As a result, there may be a notable rise in MTX-related toxicities due to prolonged, persistent, or elevated MTX plasma levels. Renal impairment was thought to be caused by the precipitation of MTX and its metabolites in the renal tubules. Previous publications stated that renal tubular enlargement and MTX-induced kidney failure are the two reasons why MTX causes renal failure (Grönroos et al. 2006; Hamed et al. 2022). Therefore, as soon as MTX treatment starts, routine monitoring of serum creatinine and plasma MTX levels is crucial to predict the onset of renal failure. Recent studies have demonstrated the use of biomarkers for kidney impairment, including kidney injury molecule-1 (KIM-1) and cystatin C in the diagnosis of kidney impairment (Hagos and Wolff 2010; van Meer et al. 2014).
Factors affecting MTX pharmacokinetics
Multiple factors contribute to the variability in MTX bioavailability among patients. Age is considered a crucial factor in both pediatric and adult populations. Delayed MTX excretion increases with age as indicated by a previous study (Zang et al. 2019; Yang et al. 2024). Additionally, total protein, albumin, and globulin levels may have some influence on MTX’s clearance because of its about 50% protein binding rate (Mei et al. 2018). Urine pH has been implicated in MTX bioavailability. Both MTX and 7-OH-MTX show limited solubility in water under acidic circumstances (pH 5–7), with 7-OH-MTX having a solubility of three to five times lower than MTX (Schofield et al. 2015). Since urine pH is directly related to renal injury, in patients receiving MTX treatments, low urine pH in the early stages of treatment is a substantial independent risk factor for MTX-induced nephrotoxicity (Kawaguchi et al. 2021). MTX is mostly taken up by cells via the SLC superfamily of transporters such as SLC19A1, SLC21, SLC22, and SLC46A and can be pumped out by different ABC (Desmoulin et al. 2012). Multiple membrane-bound proteins make up the human SLC transporter family. This family influences the development of many human diseases due to the physiological and pharmacological roles of its members. For this reason, research on SLC transporters is a crucial area for the study of therapeutic medications. In addition, gene polymorphisms in SLC transporters impact drug efficacy and toxicity (Schaller and Lauschke 2019). The solute carrier family 19 member 1 (SLC19A1) is encoded by the RFC1 gene. The second exon of RFC1 gene known as rs1051266 (80 G > A) has the most prevalent single nucleotide polymorphism (SNP), which causes arginine to histidine substitution, therefore modifying the transport capacity of MTX and subsequent pharmacokinetic profile of MTX (Giletti and Esperon 2018; Xu et al. 2022).
Adverse effects of methotrexate
Intestinal inflammation and injury are a common side effect of MTX treatment, which are caused by elevation in oxidant parameters and a decline in antioxidant status (Ozcicek et al. 2020). Additionally, various difficult toxicities associated with MTX such as testicular toxicity, which is regarded as a severe adverse effect that may result in infertility, may limit the drug’s therapeutic impact (Howard et al. 2016). Liver injury is also developed following MTX administration characterized by elevated liver function biomarkers (Bannwarth et al. 1994; Ezhilarasan 2021). As a member of category X medication, MTX is not recommended for usage during pregnancy. If this treatment is provided to a female of reproductive age, she must be aware of the possibility of teratogenesis and be instructed to use double contraception. Patients may also get mucosal ulcers when taking large doses. Other potentially severe side effects include gastrointestinal bleeding, pancreatitis, alopecia, lethargy, high body temperature, low white cell count, infections, and interstitial pneumonitis (Kremer et al. 1995; Gohar 2019; Yang et al. 2024). Generally speaking, toxicity rather than ineffectiveness is the primary reason for stopping MTX treatment (Romao et al. 2014). Periodic, meticulous, and sufficient patient monitoring appears to considerably reduce the dangers associated with the administration of MTX (Braun 2010). A better knowledge of MTX’s molecular mechanisms of action could aid in the explanation of many toxicities associated with the drug (Tian and Cronstein 2007).
Molecular mechanisms promoting intestinal injury in methotrexate injury
The exact mechanism of intestinal toxicity caused by MTX is not fully understood. However, it was reported that MTX could cause intestinal damage via producing reactive oxygen species (ROS) and transcription factor activation as NF-ĸB (Miyazono et al. 2004; Natarajan et al. 2018). NF-ĸB regulates the production of numerous cytokines and mediates cell damage, which can be activated by ROS generation (Baeuerle and Baichwal 1997; Asehnoune et al. 2004). The production of inflammatory cytokines such as TNF- α, IL-1β, and IL-6 is provoked by ROS production (Asami and Shimizu 2021). Also, MTX administration leads to inflammatory cascades involving the activation of NF-κB, with increased expression of pro-inflammatory cytokines such as IL-6 and TNF-α, followed by activation of the JAK/ STAT3 signaling (RFd et al. 2014; Kamel et al. 2022). Upon JAK/STAT phosphorylation, it translocates to the nucleus, binds with the target gene promoter region, and provokes the transcription of genes involved in the inflammatory reactions (Rawlings et al. 2004; Xin et al. 2020) (Fig. 2).
Role of inflammation in MTX-induced intestinal injury
Inflammation is a physiological reaction of the body towards external and internal stimuli (Pahwa et al. 2023). Leucocytes and plasma molecules are transported to tissues and infection sites via this process. Three main alterations take place during acute inflammation: increased capillary permeability, which permits larger serum molecules to enter the tissues, increased leukocyte migration into the tissue, and increased blood flow to the affected area (Al-Kofahi et al. 2017). The activation of macrophages and lymphocytes which leads to a coordinated cytokine response is a hallmark of chronic inflammation (Germolec et al. 2018). A key factor in intestinal injury pathogenesis is inflammation (Huang et al. 2020). Inflammatory cells against antigens release molecules called cytokines that regulate immunological and inflammatory responses. IL-1β and TNF-α, which are related to inflammation, are among these cytokines. Among these substances that raise inflammatory cytokines is MTX (Yucel et al. 2016). Numerous previous investigations have suggested that increased pro-inflammatory cytokine levels are crucial in the development of MTX-induced intestinal damage (Tunalı-Akbay et al. 2010; He et al. 2015; Kirbas et al. 2015).
Involvement of nuclear factor-kappa B (NF-ĸB) in methotrexate-induced intestinal injury
NF-kB is a key regulator of inflammation that is involved in the synthesis of inflammatory mediators and activation of pro-inflammatory cytokines (Liu et al. 2017a). A cellular NF-κB regulates the expression of numerous immune system components and modulates inflammation (Li and Verma 2002). Among these are pro-inflammatory cytokines, chemokines, and inducible enzymes such as nitric oxide synthase (iNOS) and cycloxygenase-2 (COX-2). Moreover, NF-κB regulates cytokines including IL-2 and IL-12 that affect the proliferation and differentiation of lymphocytes. Consequently, deregulation of NF-κB may lead to inflammatory conditions (Yamamoto and Gaynor 2001). The cytoplasmic NF-κB complex is bound to an inhibitor of NF-kB (IκB) and exists in an inactive state. The IκB kinase (IKK) complex is activated by TNF-α and other cell stressors through a series of intermediate stages that result in IkB phosphorylation and ubiquitination, which in turn causes IkB degradation and activates NF-κB followed by nuclear translocation to bind a specific DNA sequence (As Jr 1996; Hacker and Karin 2006; Palkowitsch et al. 2008). The transcriptional activation of NF-κB regulated genes implicated in inflammation, including IL-6 and TNF-α, is the outcome of these activities (Khongthong et al. 2019). In the same context, it is believed that the production of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 are also thought to contribute to the intestinal damage caused by MTX. Also, TNF-α inhibitors have been shown to heal mucositis in studies on humans and animals (Logan et al. 2007; Kim et al. 2009; Kirbas et al. 2015). Earlier findings have proved that MTX administration induces a series of inflammatory cascades as evident by raised NF-κB, IL-1β, and TNF-α (Sayed et al. 2021; Abd El-Ghafar et al. 2022; Hassanein et al. 2023b). Similarly, multiple previous investigations have reported that NF-κB signaling activation is highly responsible for intestinal inflammation in MTX-induced intestinal injury (Hatada et al. 2000; Jahovic et al. 2004; Zhang et al. 2022).
Role of JAK/STAT3/SOCS3 in MTX-induced intestinal injury
Many ligands use the intracellular signal transduction pathway known as JAK/STAT to activate target genes transcriptionally. When ligands bind to their receptors, they cause JAK phosphorylation, which then stimulates STAT phosphorylation, which subsequently controls the transcription of target genes which produce chemokines and pro-inflammatory cytokines (Villarino et al. 2017). There are four cytoplasmic protein tyrosine kinases in the JAK class: JAK1, JAK2, JAK3, and TYK2 (Laurence et al. 2012). Seven transcription factors are members of the STAT family: STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6 (Nicolas et al. 2013). Notably, inflammatory diseases that cause organ damage have also been associated with the JAK/STAT signaling pathway. The three primary components of the JAK/STAT signaling system are JAK, STAT, and tyrosine kinase-associated receptors (Li et al. 2015). JAK/STAT is the traditional signal pathway for multiple cytokines and growth factor production (Xin et al. 2020). When cytokines like IL-6 bind to JAK/STAT3, STAT3 becomes phosphorylated. After nuclear translocation, the phosphorylated form of STAT3 acts as a transcriptional factor that boosts the production of genes related to inflammation (Huang et al. 2016; Zhu et al. 2019). Furthermore, STATs play a crucial role in neuronal and cytokine-mediated inflammation (Kim et al. 2011). Previous studies demonstrated that MTX administration provoked JAK1 and STAT3 phosphorylation in rat models of intestinal injury and hepatotoxicity using MTX (Hassanein et al. 2021; Sherif and Al-Shaalan 2022). The regulation of the JAK/STAT system involves numerous mechanisms, one of which is the control of JAK kinase activity phosphorylation by suppressor of cytokine signaling (SOCS) proteins (Cha et al. 2015; Fouad et al. 2020; Hasan et al. 2022). The most important member of the SOCS family is SOCS3, which can block JAK/STAT3 signaling in response to mitosis, growth factors, and cytokines (Xiao et al. 2018). SOCS3 has ability to reduce JAK phosphorylation by inhibiting JAK kinase binding and competing with JAK to prevent STAT3 phosphorylation (Lin et al. 2010). Previous study showed that MTX administration showed decline in SOCS3 (de Araujo Junior et al. 2014).
Correlation between oxidative stress and MTX-induced intestinal injury
Moderate ROS is useful for multiple physiological processes such as wound healing, tissue repair, and the elimination of invasive pathogens. On the other hand, excessive ROS production leads to oxidative stress, disturbs homeostasis, and damages human tissue. It increases cellular swelling, decreases the fluidity of the cell membrane, and damages DNA, proteins, and lipids in cells (Lushchak 2014; Zhang et al. 2016). A significant source of ROS is the gastrointestinal tract, and many gastrointestinal disorders are caused by ROS. Overexposure to oxidative stress causes intestinal inflammation and mucous epithelium apoptosis, further impairing the intestinal mucosa barrier (Bhattacharyya et al. 2014). When intestinal damage is triggered by MTX, oxidative stress, a consequence of an imbalance between ROS and the body’s natural antioxidant defense mechanism, is developed and plays a crucial role (Zhang et al. 2022). ROS mediates lipid peroxidation, which leads to tissue damage development after MTX administration. This degradation of cell membranes impairs normal cellular activities (Şener et al. 2006). Additionally, according to a prior study, the antioxidant glutathione (GSH) content in cells was lowered and cytosolic peroxide was elevated following MTX treatment (Kesik et al. 2009). Multiple previous studies showed that MTX treatment altered redox status in the small intestine and increased intestinal ROS biomarkers (Miyazono et al. 2004; Hassanein et al. 2021, 2022, 2023a; Sayed et al. 2022). However, the underlying mechanism by which MTX provokes tissue injury is not yet well known, and direct toxic effects of MTX are thought to be caused by excessive generation of free radicals, causing an imbalance between free radical production and antioxidant defense, which finally results in the development of oxidative stress (Drishya et al. 2022). Tissue damage developed after MTX utilization is caused by ROS which mediates destruction of lipids resulting in a breakdown of cell membrane and disturbance of physiological processes (Şener et al. 2006). Oxidative stress causes necroptosis and apoptosis in enterocytes, as well as the destruction of the intestinal structure (Zorov et al. 2006; Pi et al. 2014). In addition, cytoskeletal proteins and other cellular proteins are damaged by an overabundance of free radicals in the intestinal epithelium. Furthermore, it increases intestinal permeability, which makes it more likely for microorganisms and antigens from the luminal environment to enter the bloodstream and increase the risk of systemic reaction syndrome (Trushina and McMurray 2007). Reactive nitrogen species (RNS) and ROS have harmful cytotoxic effects on mammalian cells in living organism. The free radicals generated during oxidative stress include non-free radical species like hydrogen peroxide (H2O2) and nitrous acid, as well as different forms of activated oxygen and nitrogen such as superoxide anion (O2•−), hydroxyl, and nitric oxide (NO) radicals (Marra et al. 2011). Oxidative stress leads to lipid peroxidation, which generates a variety of oxidative compounds, including hexanal, 4-hydroxy nonanal (4-HNE), and malondialdehyde (MDA). Although 4-HNE is the most toxic byproduct of lipid peroxidation, MDA is thought to be the most mutagenic one. Additionally, oxidative stress products such as ROS covalently modify peptide bonds or amino acid side chains resulting in protein oxidation (Unsal and Belge-Kurutaş 2017). Elevated amount of ROS leads to prolonged oxidative stress and produces a potentially hazardous environment for the cells. In normal physiologic condition, there is a balance between ROS generation and antioxidative defense mechanism in the cell. A crucial role is played by endogenous antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT) that act on O2•− and H2O2, respectively, as well as glutathione peroxidase (Gpx) that uses GSH as co-substrate (Fu and Chung 2018).
Nuclear factor erythroid-2-related factor 2 (Nrf2)-Kelch-like ECH-associated protein 1 (Keap1) pathway and intestinal injury by MTX
The primary regulator of cellular responses to external stressors is nuclear factor erythroid-2-related factor 2 (Nrf2) (Kobayashi et al. 2004). The nuclear factor erythroid-2-related factor 2 gene is responsible for encoding antioxidants and detoxification enzymes providing a redox sensing system (Wang et al. 2008). Kelch-like ECH-associated protein 1 (KEAP1) is a natural inhibitor of Nrf2 that negatively regulates its activity by proteasomal degradation (Singh et al. 2006). Following xenobiotic exposure, the Nrf2/Keap1 pathway is activated, releasing Nrf2 and causing it to translocate into the nucleus where it forms a heterodimer with its partner sMAF oncogene homolog. Then, it binds to the antioxidant response element (ARE) sequences regulating several targeted genes such as glutathione S-transferase (Gst) and heme oxygenase-1 (HO-1) (Taguchi et al. 2011).
The genes that encode drug-metabolizing enzymes and transporters, antioxidant enzymes, and heme and iron metabolic enzymes are among Nrf2’s target genes (Suzuki et al. 2013). The intestine and lung, two detoxifying organs or tissues that directly oppose the environment, have notably high Nrf2 expression levels (Kobayashi et al. 2004).
In a dose- and time-dependent manner, hyperactivation of Nrf2 diminished oxidative stress by ameliorating cell apoptosis and improving the redox state of the cell (Song et al. 2017). Several experimental models have been used to study Nrf2’s capacity to maintain the intestinal barrier, including Salmonella typhi infections (Theiss et al. 2009), colitis caused by dextran sodium sulfate (Theiss et al. 2009; Li et al. 2018), intestinal ischemic-reperfusion (Chi et al. 2015; Han et al. 2016b), intestinal mucosa damage, malfunction of the epithelial barrier brought on by traumatic brain injury (Liu et al. 2017b), and intestinal burn (Chen et al. 2016). In the same context, the previous publication reported the involvement of Nrf2 in MTX-induced intestinal injury (Katturajan and Evan Prince 2023).
By activating transcription factors like NF-κB and activator protein 1 (AP-1) and upregulating kinases like phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinases (MAPKs), ROS generation can cause pro-inflammatory responses (Chen and Kunsch 2004). The production of ROS has the potential to trigger immune cell activation and persistent inflammation. However, persistent inflammation can also worsen the production of ROS, creating a vicious circle (Chen and Kunsch 2004). It has been proposed that reducing ROS production can lessen inflammation. Numerous investigations have indicated that there is a strong correlation between Nrf2 and NF-κB pathways. To test it, colitis was induced in Nrf2-deficient animals by administering dextran sulfate sodium treatment. Also, mice lacking Nrf2 showed higher levels of inflammation than wild-type mice (Khor et al. 2006). In conclusion, Nrf2 activation can reduce intestinal inflammation due to direct control of inflammatory pathways by suppression of ROS production (Wen et al. 2019).
Involvement of peroxisome proliferator-activated receptor-gamma (PPAR-γ) in MTX-induced intestinal toxicity
Peroxisome proliferator-activated receptors (PPARs) belong to the nuclear hormone receptor superfamily and are ligand-dependent transcription factors. They are essential for the metabolism of carbohydrates and lipids (Wahli et al. 1995). Vertebrates have been found to have the two PPAR isotypes: PPAR-α and PPAR-γ. The liver, kidney, testes, heart, pancreas, and smooth muscle all have high levels of PPAR-α isoform expression. For instance, adipose tissue has high levels of PPAR-γ expression, and the intestines, particularly the colon, also contain it (Auboeuf et al. 1997). In addition to being a powerful regulator of energy balance and systemic as well as cellular metabolism, PPARα also suppresses a number of inflammatory responses (Liu et al. 2018). In both white and brown adipose tissue, PPAR-γ is highly expressed and is essential for controlling lipid production, energy balance, and adipogenesis. Additionally, it is expressed in the intestines, liver, kidneys, brain, immune system, and muscles (Willson et al. 2001; Moreno et al. 2004; Grygiel-Górniak 2014). PPARs move into the nucleus after interacting with agonists, where they heterodimerize with the retinoid X receptor (RXR) to perform their function. The heterodimers stimulate the transcription of the targeted genes by binding to sequence-specific PPAR response elements (PPREs) (Berger and Moller 2002). Interestingly, it is widely known that PPAR-γ is a powerful inhibitor of ROS and inflammation (Stafeev et al. 2015). PPAR-γ carries out numerous biological functions. Its conformation changes upon activation preventing the production of pro-inflammatory mediators, which in turn prevents a range of inflammatory responses (Wang et al. 2016). The reduction of NF-kB, STAT1, and AP-1 transcriptional activity is one of the PPAR-γ anti-inflammatory actions (Ricote et al. 1999). Actually, a number of studies have also shown a metabolic advantage associated with the anti-inflammatory effects of PPAR-γ targeting (Hevener et al. 2007). It is interesting to note that PPAR-γ is a significant nuclear receptor whose beneficial antioxidant and anti-inflammatory properties have led to the investigation of a variety of disorders (Korbecki et al. 2019). Previous literature has indicated that the expression of the antioxidant defense can be induced by activated PPAR-γ (Girnun et al. 2002; Chung et al. 2009) while inhibiting inflammatory cytokine productions (Vandewalle et al. 2008). Previous studies showed that a decreased level of PPAR-γ is associated with oxidative stress development in a rat model of duodenal injury induced by MTX (Sayed et al. 2022; Mansoury et al. 2023).
Role of silent information regulator-1 (SIRT1) in MTX-induced intestinal toxicity
Among the histone deacetylases which are referred to as sirtuin1, is the silent information regulator-1 (SIRT1) protein. Significantly, SIRT1 is critical for controlling oxidative stress and mitochondrial metabolism. By controlling antioxidant genes through the FoxO3a/proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1α) complex, SIRT1 prevents the generation of superoxide (Wang et al. 2020c). Additionally, recent studies suggested that SIRT1 is an essential regulator of intestinal barrier function (Tanno et al. 2007; Tao et al. 2010). Moreover, SIRT1 activation significantly ameliorated colitis induced by dextran sulfate sodium in mice (Kwon et al. 2017). It has been demonstrated to block the NF-κB signaling suppressing the inflammatory response. According to recent reports, SIRT1 contributes to cellular lifespan extension, resistance to oxidative stress, and repair of DNA damage (Elshazly et al. 2020; Gao et al. 2021). Mice with intestinal deletion of SIRT1 have been shown to exhibit microbiota dysbiosis and aberrant activation of the inflammatory response (Wellman et al. 2017). Previous publications have demonstrated that oxidative stress significantly decreased SIRT1 activity in previously experimental models. (DiNicolantonio et al. 2022). High ROS can suppress SIRT1 function by causing oxidative changes in its cysteine residues (Salminen et al. 2013). In addition, SIRT1 induces the inhibition of NF-κB and additional pro-inflammatory mediators (Shao et al. 2014). Previous publications have demonstrated the involvement of SIRT1 in MTX-provoked intestinal injury (Sayed et al. 2022; Katturajan and Evan Prince 2023; Abd-Alhameed et al. 2024).
Therapeutic protection against methotrexate-induced intestinal injury
Omega-3 polyunsaturated fatty acids
Over the past decades, polyunsaturated fatty acids (PUFAs) have become a topic of interest for the public and scientific community due to their involvement in numerous metabolic and physiological conditions (Palmquist 2009). Fatty fish and seafood are the main sources of them (Han et al. 2016a). Interestingly, omega-3 FAs anti-inflammatory and antioxidant properties proved their effectiveness in both preventing and treating a wide range of illnesses (Swanson et al. 2012; Scorletti and Byrne 2013; Firat et al. 2017; Oscarsson and Hurt-Camejo 2017; Karageorgou et al. 2023). Additionally, omega-3 PUFAs exhibited potential protective effect against MTX-induced apoptosis in spleen (Elsayed et al. 2021) and intestinal mucosa (Koppelmann et al. 2021) as well as acute kidney injury induced by lipopolysaccharide (Li et al. 2021b). Interestingly, omega-3 PUFAs were evaluated in rat models of intestinal damage by MTX, and this study demonstrated that omega-3 PUFAs exhibited the ability to prevent intestinal damage and stimulate intestinal recovery. Besides, MTX + omega-3 PUFA-treated rats showed a significant decrease in enterocyte apoptosis together with reduced numbers of macrophages in conjunction with lower levels of COX-2, TNF-α, and NF-κB in the mucosa of treated rats (Koppelmann et al. 2021). This study found that omega-3 PUFAs may be used as a novel therapy for attenuating MTX-induced intestinal injury through its antioxidant, anti-inflammatory, and antiapoptotic effect.
Taurine
Taurine, also known as 2-aminoethanesulfonic acid, is a common organic substance found in animal tissues. It accounts for 0.1% of the human body weight and is primarily found in the large intestine as well as the main component of bile (Ronalds 2019). Taurine plays a vital role in many processes, including control of osmotic pressure, the stabilization of membranes, reproduction, inflammation, and the regulation of heart muscle. Numerous studies have proved that taurine is a promising agent due to its ability to overcome oxidative stress and inflammation. It can be used to protect against a wide range of conditions in several organ systems, including the skeletal, muscular, cardiovascular, respiratory, and endocrine systems (Ahmed 2023). Taurine plays a crucial role in protecting against nervous system diseases including Parkinson’s and Alzheimer’s (Jakaria et al. 2019). Molecular studies have indicated that it may act as a neuroprotectant against stroke. In a diabetic mouse model, it reduced oxidative stress-induced neuropathy by triggering antioxidative defense signals (Agca et al. 2014). Obviously, previous publications demonstrated the protective effect of taurine against MTX-induced intestinal injury through a variety of mechanisms following careful examination. The effective modulation of cytoglobin and Keap1/Nrf2/HO-1 signals mediated its potent antioxidant effects. The inhibition of the NF-κB/iNOS signal suggests its anti-inflammatory effects. Intestinal proliferating cell nuclear antigen (PCNA) and caspase-3 suppression mediate antiapoptotic and antiproliferative effects (Hassanein et al. 2022). This study explained that taurine may be used as a promising therapy in mitigating intestinal damage provoked by MTX through the regulation of oxidative stress, inflammation, apoptosis, and proliferation.
Umbelliferone
A naturally occurring member of the coumarin family, umbelliferone (UMB) or 7-hydroxycoumarin, is present in a wide variety of plants, including garden angelica, coriander, and carrots (Mazimba 2017). Numerous investigations have determined that UMB possesses biological properties, including anti-inflammatory (Navarro-García et al. 2011), antioxidant (Hoult and Payá 1996; Cruz et al. 2020), and anticancer (Lopez-Gonzalez et al. 2004) effects. In testicular dysfunction in diabetic heavy metal-treated mice (Allam et al. 2022; Alotaibi et al. 2023), liver injury (Shalkami et al. 2021), kidney injury (Sami et al. 2022), and liver fibrosis (Park et al. 2023b), UMB showed potent antioxidant and anti-inflammatory properties as well as decreased cell damage. According to a study reported previously (Jagadeesh et al. 2016), UMB improved heart function and reduced oxidative stress and infarct size in rats. Previous study proved the promising protective effect of UMB against MTX-induced intestinal damage by markedly improved oxidant/antioxidant status, as shown by the parallel decrease in MDA contents and the elevation of Nrf2, SOD, HO-1, and GSH levels. Additionally, it reduced the number of inflammatory cascades by inhibiting STAT3, NF-κB, IL-6, and TNF-α levels. Furthermore, the expression of Wnt and β-catenin was dramatically increased by UMB (Hassanein et al. 2023a). According to these results, UMB might be applied as a possible adjuvant treatment in MTX chemotherapy regimens to overcome intestinal injury caused by MTX through the regulation of oxidative stress and inflammatory cascades.
Vinpocetine
Ethyl apovincaminate, also known as vinpocetine, is a nootropic substance that has been intended to manage neurological illnesses related to cerebrovascular diseases. It is a synthetic derivative of the alkaloid vincamine, which is taken from the leaves of the periwinkle plant (Mohammed et al. 2023). Moreover, vinpocetine has a strong antioxidant impact by scavenging free radicals and a strong anti-inflammatory effect by directly inhibiting IKK (Abdel-Salam et al. 2016; Nadeem et al. 2018; Zhang et al. 2018). Additionally, previous study has demonstrated the increase in cerebrovascular flow by vinpocetine in individuals with cerebrovascular illness (Patyar et al. 2011). Vinpocetine’s anti-neuroinflammatory and antioxidant pathways have been suggested to be involved in its neuroprotective impact on rotenone-induced Parkinson’s disease (Ishola et al. 2023). Vinpocetine has been studied in a rat model of duodenal injury by MTX, and this study showed that the injection of vinpocetine retained the normal histology of the crypt and villous while attenuating the dramatic histological alterations caused by MTX. Through the upregulation of intestinal Nrf2 and HO-1 expression, vinpocetine dramatically reduced oxidative stress damage. By lowering IL-1β and TNF-α levels and downregulating the expressions of NF-κB, interferon regulatory factor3 (IRF3), p-JAK-1/p-STAT-3, and vinpocetine reduced inflammation. Moreover, vinpocetine efficiently inhibited caspase-8, RIPK1, RIPK3, and MLKL to counteract intestinal necroptosis (Tashkandi et al. 2023). Due to these favorable effects, vinpocetine can be used as a complementary therapy with MTX to counteract apoptosis, inflammation, and oxidative stress by MTX.
Perindopril
Through processes involving angiotensin II, perindopril (PER), a typical angiotensin-converting enzyme inhibitor (ACEI), has been shown to be useful in a number of cardiovascular disorders (Ancion et al. 2019). PER has also been demonstrated to have antiapoptotic, anti-inflammatory, and antioxidant properties (Varin et al. 2000, Kobara et al. 2003). Earlier study has shown that PER has a potent antioxidant and anti-inflammatory activity which be helpful in treating acute kidney injury associated with sepsis (Ali et al. 2016; Kostakoglu et al. 2020). Previous research has also demonstrated that PER can reduce drug-induced kidney damage due to its antioxidant and anti-inflammatory properties (Tang et al. 2008; Shalkami et al. 2018). Preliminary investigation also showed gastroprotective effect of perindopril through counteracting oxidative stress and inflammation induced by indomethacin in a rat model of gastric injury (Mohamed et al. 2022). Previous publication has demonstrated the potential protective effect of perindopril on intestinal injury induced by MTX. This study showed that perindopril preserved the goblet cells in the villi/crypts and reduced the histological abnormalities, indicating that the intestinal injury had been attenuated. Additionally, PER reduced intestinal MDA and increased SOD activity and GSH content along with PPAR-γ and SIRT1 cytoprotective signals to attenuate the pro-oxidant processes. These favorable effects were also associated with upregulating angiotensin (1–7) and anti-inflammatory cytokine IL-10 while downregulating the production of pro-inflammatory cytokines IL-6, IL-1β, and TNF-α. Besides, in rats with inflamed intestines, PER downregulated the toll-like receptor 4 (TLR4), NF-κB, and c-Fos/c-Jun pathways at the molecular level (Sayed et al. 2022). In conclusion, PER significantly reduced MTX-induced intestinal damage by inhibiting inflammatory pathways and increasing the antioxidant cytoprotective signals.
Rutin
Rutin is one of the main flavonoid glycosides present in fruits and fruit peels, mainly in citrus fruits such as lemons and oranges (Nafees et al. 2015; Çelik et al. 2020). It possesses several pharmacological activities, including the ability to effectively scavenge superoxide radicals and act as an immunomodulator, anti-inflammatory, antioxidant, antihypertensive, and anti-carcinogenic (Nafees et al. 2015; Caglayan et al. 2019; Kandemir et al. 2020). The main pharmacological effects and underlying mechanism of action of rutin contribute to its antioxidant capacity through the Nrf2/ARE and anti-inflammatory properties due to NF-κB, COX-2, IL-6, and TNF-α suppression. It inhibits caspase-3 and enhances B-cell lymphoma 2 (Bcl-2) suggesting its antiapoptotic effect (Janbaz et al. 2002; Nafees et al. 2015). It has been shown that rutin can ameliorate liver and/or kidney injury induced by different agents such as lead acetate (Ansar et al. 2016), acrylamide (Ahmed and Ibrahim Laila 2018), and carbon tetrachloride (Hafez et al. 2015). According to a previous investigation, rutin protects the kidneys in diabetic nephropathy (Kamalakkannan and Prince 2006) and ischemic/reperfusion renal damage (Korkmaz and Kolankaya 2013). Rutin has been shown to inhibit renal apoptosis and inflammation caused by cisplatin by lowering the expression of caspase-3, as well as TNF-α,and NF-κB (Tambağ et al. 2021). Rutin has been evaluated in a previous study of intestinal toxicity induced by MTX, and this study showed its ability to attenuate intestinal oxidative stress changes by lowering intestinal MDA and boosting GSH content and SOD activity. Moreover, administration of rutin attenuated MTX-induced intestinal inflammation, as proved by decreased IL-2 and increased IL-4 and IL-10. Additionally, rutin was found to inhibit the enzymatic activity of COX and lipoxygenase.
It can be concluded that rutin, in a dose-dependent manner, has significant physiological, immunological, and biochemical protection against MTX-induced intestinal injury (Gautam et al. 2016). The immunoregulatory and free radical scavenging potential activity could be thought of as the explanations for rutin’s activities.
Hesperidin
Flavonoids, which are easily derived from various vegetables and fruits and possess anti-inflammatory and antiapoptotic effects in addition to their anti-autophagic properties, have gained more attention in recent times. Hesperidin (HES), a flavanone group member, is one of these compounds (Semis et al. 2021). Citrus fruits, including lemon, orange, and grapefruit, are a good source of this natural antioxidant compound (Yurtal et al. 2020; Patel and Shah 2021). HES exhibited antiapoptotic, anti-inflammatory, vasoprotective, and anti-carcinogenic properties together with its antioxidant activity, with no known adverse effects (Çetin et al. 2016; Sheikhbahaei et al. 2016; El et al. 2017). According to reports, HES scavenges ROS, chelates metal ions, and guards against lipid peroxidation to avoid oxidative damage and cell death (Polat et al. 2016; Iskender et al. 2017). Previous studies have shown the protective effect of HES against MTX-induced hepatotoxicity (Abdelaziz et al. 2020), experimental ischemia/reperfusion testicular injury in rats (Celik et al. 2016), nephrotoxicity and hepatotoxicity induced by sodium arsenite (Turk et al. 2019), paclitaxel-induced peripheral neuropathy in rats (Semis et al. 2021), and renal ischemia-reperfusion injury in rats (Meng et al. 2020). A potential experimental investigation has assessed the protective effect of HES against intestinal damage provoked by MTX using histopathological and immunohistochemical techniques. Pretreatment with HES attenuated intestinal injuries evidenced by enhancing intestinal scoring damage and crypt injury. Additionally, administration of HES counteracted intestinal oxidative stress changes by lowering intestinal myeloperoxidase concentration. Moreover, treatment with HES attenuated MTX-induced intestinal inflammation, as proved by inhibiting INOS and IL-8 level immunostaining (Acipayam et al. 2013). In conclusion, HES significantly showed notable amelioration of intestinal damage induced by MTX through its powerful antioxidant and anti-inflammatory effect.
Lycopene
Tomatoes and other red fruits have a high concentration of the red pigment lycopene. Many double bonds in lycopene’s chemical structure play a significant part in scavenging ROS (Abdel-Daim et al. 2019; Ibrahim et al. 2022). Because of its 11 conjugated double bonds, lycopene exhibited the highest antioxidant activity among carotenoids and phytochemicals (Saini et al. 2020). Lycopene has numerous pharmacological properties including potent and effective anti-inflammatory, immunostimulant, antibacterial, and anti-mutagenic properties (Müller et al. 2011). In addition, lycopene exhibits chemo-preventive properties against some types of cancer (Huang and Hu 2011). It was found to have a potent free radical scavenging effect during severe stressful conditions. Eating tomatoes or tomato-derived products is frequently associated with lower levels of oxidative damage to proteins, lipids, and DNA due to higher amounts of circulating lycopene (Palabiyik et al. 2013). Furthermore, it is well known that dietary lycopene supplementation shields the animal intestine’s structure and tissue from harmful events when it comes into touch with pathogens, poisons, or any other foreign antigen (Sarker et al. 2021). Previous investigations showed that lycopene administration exhibited significant protection against intestinal injury provoked by radiation in rats (Anwar et al. 2013). Due to its strong scavenging activity of free radicals, lycopene has a powerful ability to protect the kidney, liver, and nervous system from oxidative stress (Zhang et al. 2020). Lycopene has recently shown obvious neuroprotective effects in several conditions involving neuroinflammatory conditions. These advantageous outcomes were due to NF-κB suppression, the maintenance of mitochondrial integrity, and the reduction of apoptosis in neurons (El Morsy and Ahmed 2020).
Lycopene was studied in a previous study investigating its protective effect against MTX-provoked intestinal injury, and this study showed that when lycopene was administered to the MTX group, the small intestinal histological damage showed a considerable recovery. Additionally, the intestinal levels of IL-1β, total oxidant status (TOS), and oxidative stress index (OSI) were dramatically reduced (Yucel et al. 2016). Because lycopene counteracts oxidative stress, inflammation, and NF-κB activation created by MTX, it may be an excellent adjuvant therapy taken with MTX.
Quercetin
Of all the flavonoids, quercetin (3, 39, 49, 5, 7-pentahydroxyflavone) is the most widely distributed. Apples, potatoes, soybeans, and other fruits and vegetables are rich sources of quercetin (Mao et al. 2018). Strong cytoprotective and antioxidative properties of quercetin help in inhibiting endothelial cell apoptosis induced by oxidants (Choi et al. 2003). Besides, by stopping lipid peroxidation and scavenging oxygen free radicals, quercetin inhibits oxidative damage and cell death (Wang et al. 2020a). Quercetin is a powerful antioxidant that protects against ROS and has been shown to have remarkable protective benefits against diabetes, cardiovascular disease, inflammation, cancer, and damage to nerves and the eyes (Carrillo-Martinez et al. 2024). Previous publication has investigated the ability of quercetin to attenuate small intestine damage and improve intestinal recovery in MTX-induced intestinal mucositis in rats. When quercetin was administered to rats treated with MTX, a significant reduction in the intestinal injury score, a significant increase in the intestinal and mucosal weight in the ileum and jejunum, and an increase in the ileum’s protein content and villus height were observed in comparison to the MTX group (Sukhotnik et al. 2018).
Apocynin
Apocynin (APO) (4-hydroxy-3-methoxyacetophenone) is a natural organic methoxy-substituted catechol compound that acts as an antioxidant. It is separated from Apocynum cannabinum root and Picrorhiza kurroa Royle ex Benth, a traditional medicinal plant, that belongs to the Scrophulariaceae family (Nwokocha et al. 2020). The primary enzyme that produces ROS is nicotinamide adenine dinucleotide phosphate oxidase (NOX), and blocking this enzyme offers a significant therapeutic target for the management of numerous illnesses (Auten et al. 2009; Datta et al. 2015). APO efficiently blocks NOX in activated leukocytes to stop ROS generation (Stefanska and Pawliczak 2008). APO has been shown in numerous animal and cell culture experiments to decrease neutrophil chemotaxis and neutrophil oxidative burst, which in turn reduces neutrophil-mediated cell damage (Stolk et al. 1994; Impellizzeri et al. 2011). According to previous investigations, APO ameliorated lung damage by reducing lipid peroxidation, inhibiting NOX expression and activity, blocking the NF-κB pathway, and suppressing the transcription of pro-inflammatory cytokines in lung tissue (Kim et al. 2012; Choi et al. 2017). A previous study investigated the protective role of APO against MTX-induced intestinal mucositis. The conserved histology of goblet cells (villi and crypts) indicates that APO preserved the histological structure of the duodenal mucosa. Besides, APO reduced intestinal oxidative stress by decreasing intestinal MDA and increasing SOD activity and GSH content. APO exhibited powerful anti-inflammatory by inhibiting the production of NF-κB mRNA and decreasing pro-inflammatory cytokine levels together with upregulating anti-inflammatory PPAR-γ proteins. Furthermore, the intestinal mucosa of rats that received APO + MTX revealed weak positive immunological staining for cleaved caspase-3 (Mansoury et al. 2023). The findings suggest that APO may be used as a potential treatment drug to stop mucositis caused by MTX due to counteracting oxidative stress, inflammatory, and apoptotic pathways.
Lactobacillus
A genus of gram-positive anaerobic or microaerophilic, rod-shaped, non-spore-forming bacteria is called Lactobacillus. They are an important component of the microbiota in humans and can be found in the urinary, genial, and digestive systems (Wanchao et al. 2018). Moreover, Lactobacillus scavenges ROS to prevent oxidative damage development (Kong et al. 2020). Besides Lactobacillus administration diminishes ROS such as hydroxyl radicals, superoxide anions, and peroxide radicals (Wang et al. 2017). Research conducted on pigs has demonstrated that a diet containing Lactobacillus raises muscle SOD, CAT, and serum SOD (Wang et al. 2009). Lactobacillus has been shown to have a variety of anti-carcinogenic effects due to its ability to antagonize proliferation, apoptosis, and oxidative stress (Nowak et al. 2019). Lactobacillus shields the intestines against several intestinal damage models (Jian et al. 2022; Hassanein et al. 2023a). In the same context, Lactobacillus showed a protective effect against a rat model of 5-aminosalicylic-induced ulcerative colitis through the Nrf2/Ho-1 pathway and gut microbiota modulation (El-Baz et al. 2020). A previous publication has proved the intestinal protective effect of Lactobacillus acidophilus LB strain (LB) against MTX-induced intestinal injury. Pretreatment with Lactobacillus attenuated intestinal injury as evidenced by improvement of intestinal histopathology. Lactobacillus treatment attenuated intestinal oxidative stress changes by lowering intestinal MDA and boosting GSH content, SOD3 activity, Nrf2, and OH-1. Moreover, administration of Lactobacillus attenuated MTX-induced intestinal inflammation, as proved by inhibiting TNF-α, IL-6, STAT3, and NF-κB (Hassanein et al. 2023a). In conclusion, by restoring the oxidant/antioxidant balance and reducing the inflammatory burden, a pretreatment regimen with lactobacillus may be a potential therapeutic approach for attenuating intestinal injury caused by MTX.
Berberine and zinc
Berberine, a well-known natural isoquinoline alkaloid, is found in a variety of plants, such as Coptis and Berberis (Cicero and Baggioni 2016). It is interesting to note that traditional medicine uses berberine to treat intestinal disorders and diarrhea (Tan et al. 2019). It also has anti-inflammatory, antioxidant, and anticancer properties (Tan et al. 2011; Deng et al. 2019; Hassanein et al. 2019). Significantly, it was documented that berberine prevented ulcerative colitis provoked by dextran sulfate sodium (Zhu et al. 2019). Obviously, berberine has been shown to have a potent anti-inflammatory impact in cases of severe abdominal infection and to mitigate intestinal mucosa injury due to stressful conditions (Wang et al. 2020b). Zinc (Zn) is a vital trace element that has anti-inflammatory and antioxidant effects and is involved in numerous biological processes (Haase et al. 2008; Marreiro et al. 2017). Previous publications have shown that Zn attains a beneficial protective effect on intestinal injury conditions. They found that Zn in the intestinal lumen can decrease sensitivity to damage and increase mucosal repair and restoration. Besides, they reported that Zn supplementation improved the gut’s ability to heal from MTX-induced injury (Tran et al. 2003; Musa et al. 2015). A previous study showed that berberine and/or Zn exhibited a notable protective effect against MTX-induced intestinal toxicity. Berberine and/or Zn ameliorated oxidative stress and enhanced changes in SIRT1, Nrf2, forkhead box-O3 (FOXO-3), JAK1, and STAT3 (Hassanein et al. 2021). Berberine and Zn may be possible medications for the intestinal damage management produced by MTX by altering the signaling pathways involved in oxidative stress and inflammation.
Nifuroxazide
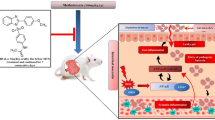
Nifuroxazide (NIF) is a highly safe oral antidiarrheal antibiotic that has been approved for the treatment of several gastrointestinal infections (Hassan et al. 2021). NIF, an antidiarrheal antibiotic, demonstrated efficacious suppression of STAT3 activation in cell lines of colorectal cancer, multiple myeloma, colon ulcer, and diabetic kidney tissues (Althagafy et al. 2023). Previous research demonstrated that NIF inhibited NF-κB signaling in liver failure induced by thioacetamide in rats (Khodir and Said 2020) and acetic acid-induced ulcerative colitis (Yousra et al. 2020). Remarkably, NIF also improved rat diabetic nephropathy by inhibiting pro-inflammatory cytokine production, oxidative stress, and NF-κB activation in diabetic kidneys (Elsherbiny et al. 2018). Regarding the impact of NIF on MTX-induced intestinal injury, NIF exhibited potent antioxidant benefits against MTX-provoked intestinal injury by controlling PPAR-γ, SIRT1, and Nrf2 redox-sensitive signals expression (Abd-Alhameed et al. 2024). In addition, intestinal inflammation was reduced by NIF due to suppression of NF-κB protein expression, downregulation of JAK1/STAT3 phosphorylation, and reducing the release of pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α. Furthermore, the histological analysis showed that NIF decreased the invasion of inflammatory cells, maintained the goblet cells, and improved the pathological alterations in the intestines. Consequently, NIF may be a good option as MTX adjuvant therapy via counteracting oxidative stress, inflammation, and NF-κB activation caused by MTX.
Conclusions and perspectives
MTX is a well-known cytotoxic medication that is frequently used for managing autoimmune diseases and malignancies. Using MTX may be associated with intestinal mucous membrane damage as well as mucositis, which impairs the ability of patients to tolerate treatment and disturbs their nutritional status. Due to limited treatment options and severe adverse effects associated with MTX, multiple approaches strongly need to be investigated to counteract these severe adverse effects. By inducing lipid peroxidation and excessive ROS, MTX induces a series of oxidative stress of the intestinal mucosal membrane. Besides, it triggers the release of pro-inflammatory cytokines like NF-kB, IL-6, IL-1β, and TNF-α as well as the activation of many pro-inflammatory signaling pathways via ROS-driven mechanisms. Various molecular pathways are also involved in MTX-induced intestinal injury including JAK/STAT3/SOCS3, Nrf2/OH-1, PPAR-γ, and SIRT1. Thus, individuals with rheumatoid arthritis, psoriasis, and cancer who take MTX need to be monitored for intestinal injury. Multiple compounds such as omega-3 polyunsaturated fatty acids, taurine, umbelliferone, vinpocetine, perindopril, rutin, hesperidin, lycopene, quercetin, apocynin, lactobacillus, berberine, zinc, and nifuroxazide have been studied in previous publications and were reported to have potential protective effects in ameliorating MTX-provoked intestinal injury. As a result, novel treatment approaches for managing and alleviating MTX-induced intestinal injury will soon be required. Possible molecular mechanisms involved in MTX-induced intestinal injury and mechanisms of actions of protective agents are graphically illustrated in Fig. 3.
Hence, more investigations are needed to assess other signaling molecular pathways involved in MTX-induced intestinal injury to develop novel strategies for amelioration of intestinal injury induced by MTX. Also, further clinical studies should be done to emphasize the potential uses of previously mentioned protective agents as a potential supplementary therapy for preventing MTX-provoked intestinal injury.
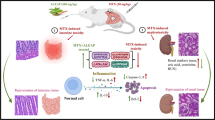
Detailed mechanism of signaling molecular pathways involved in MTX-induced intestinal injury. MTX-induced intestinal injury by increasing oxidative stress characterized by decreasing the activation of antioxidants GSH and SOD and increasing pro-oxidant MDA mediated by the downregulation of Nrf2/HO-1, PPAR-γ, and SIRT1. Also, MTX administration enhanced inflammation characterized by increasing pro-inflammatory cytokines TNF-α and IL-6 mediated by the upregulation of NF-κB and JAK/STAT3 phosphorylation and decreasing SOCS3. Furthermore, it increased apoptosis characterized by an elevation of cleaved caspase-3 and caspase-8. Abbreviations: GSH, glutathione; IL-6, interleukin-6; JAK/STAT3, Janus kinase/signal transducer and activator of transcription3; MDA, malondialdehyde; MTX, methotrexate; Nrf2/HO-1, nuclear factor erythroid-2-related factor 2/heme oxygenase-1; NF-κB, nuclear factor-kappa B; PPAR-γ, peroxisome proliferator-activated receptor-gamma; SOD, superoxide dismutase; SOCS3, suppressor of cytokine signaling3; SIRT1, silent information regulator-1; TNF-α, tumor necrosis factor-alpha
Data availability
No datasets were generated or analysed during the current study.
References
Abd El-Ghafar OAM, Hassanein EHM, Ali FEM, Omar ZMM, Rashwan EK, Mohammedsaleh ZM, Sayed AM (2022) Hepatoprotective effect of acetovanillone against methotrexate hepatotoxicity: role of Keap-1/Nrf2/ARE, IL6/STAT-3, and NF-κB/AP-1 signaling pathways. Phytother Res 36:488–505
Abd-Alhameed EK, Azouz AA, Abo-Youssef AM, Ali FE (2024) The enteroprotective effect of nifuroxazide against methotrexate-induced intestinal injury involves co-activation of PPAR-γ, SIRT1, Nrf2, and suppression of NF-κB and JAK1/STAT3 signals. Int Immunopharmacol 127:111298
Abdel-Daim MM, Eissa IA, Abdeen A, Abdel-Latif HM, Ismail M, Dawood MA, Hassan AM (2019) Lycopene and resveratrol ameliorate zinc oxide nanoparticles-induced oxidative stress in Nile tilapia, Oreochromis niloticus. Environ Toxicol Pharmacol 69:44–50
Abdel-Salam OM, Hamdy SM, Seadawy SAM, Galal AF, Abouelfadl DM, Atrees SS (2016) Effect of piracetam, vincamine, vinpocetine, and donepezil on oxidative stress and neurodegeneration induced by aluminum chloride in rats. Comp Clin Pathol 25:305–318
Abdelaziz RM, Abdelazem AZ, Hashem KS, Attia YA (2020) Protective effects of hesperidin against MTX-induced hepatotoxicity in male albino rats. Naunyn Schmiedebergs Arch Pharmacol 393:1405–1417
Abolmaali SS, Tamaddon AM, Dinarvand R (2013) A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother Pharmacol 71:1115–1130
Acipayam C, Bayram I, Daglioglu K, Doran F, Yilmaz S, Sezgin G, Totan Ateş B, Ozkan A, Tanyeli A (2013) The protective effect of hesperidin on methotrexate-induced intestinal epithelial damage in rats: an experimental study. Med Principles Pract 23:45–52
Agca CA, Tuzcu M, Hayirli A, Sahin K (2014) Taurine ameliorates neuropathy via regulating NF-κB and Nrf2/HO-1 signaling cascades in diabetic rats. Food Chem Toxicol 71:116–121
Ahmed AOH (2023) A brief insight about taurine and its antioxidant effects. Tobacco Regulatory Science (TRS) 9:2929–2936
Ahmed MM, Ibrahim Laila IM (2018) Rutin ameliorates acrylamide-induced hepatotoxicity and biochemical disturbance in male albino rats. Scientific Journal of October 6 University 4:8–13
Al-Kofahi M, Yun JW, Minagar A, Alexander JS (2017) Anatomy and roles of lymphatics in inflammatory diseases. Clin Experimental Neuroimmunol 8:199–214
Ali MRA-A, Abo-Youssef AMH, Messiha BAS, Khattab MM (2016) Tempol and Perindopril protect against lipopolysaccharide-induced cognition impairment and amyloidogenesis by modulating brain-derived neurotropic factor, neuroinflammation and oxido-nitrosative stress. Naunyn Schmiedebergs Arch Pharmacol 389:637–656
Allam MA, Khowailed AA, Elattar S, Mahmoud AM (2022) Umbelliferone ameliorates oxidative stress and testicular injury, improves steroidogenesis and upregulates peroxisome proliferator-activated receptor gamma in type 2 diabetic rats. J Pharm Pharmacol 74:573–584
Althagafy HS, El-Aziz MKA, Ibrahim IM, Abd-Alhameed EK, Hassanein EHM (2023) Pharmacological updates of nifuroxazide: promising preclinical effects and the underlying molecular mechanisms. Eur J Pharmacol 951:175776
Alotaibi MF, Al-Joufi F, Abou Seif HS, Alzoghaibi MA, Djouhri L, Ahmeda AF, Mahmoud AM (2023) Umbelliferone Inhibits Spermatogenic Defects and Testicular Injury in Lead-Intoxicated Rats by Suppressing Oxidative Stress and Inflammation, and Improving Nrf2/HO-1 Signaling [Retraction]. Drug Des Dev Ther 17:1153–1154
Ancion A, Tridetti J, Nguyen Trung M-L, Oury C, Lancellotti P (2019) A review of the role of bradykinin and nitric oxide in the cardioprotective action of angiotensin-converting enzyme inhibitors: focus on perindopril. Cardiol Therapy 8:179–191
Ansar S, Hamed S, AlGhosoon H, AlSaedan R, Iqbal M (2016) The protective effect of rutin against renal toxicity induced by lead acetate. Toxin Reviews 35:58–62
Anwar M, Nanda N, Bhatia A, Akhtar R, Mahmood S (2013) Effect of antioxidant supplementation on digestive enzymes in radiation induced intestinal damage in rats. Int J Radiat Biol 89:1061–1070
As B Jr (1996) The NF-κB and l-κB proteins: new discoveries and insights. Annu Rev Immunol 14:649
Asami J, Shimizu T (2021) Structural and functional understanding of the toll-like receptors. Protein Sci 30:761–772
Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E (2004) Involvement of reactive oxygen species in toll-like receptor 4-dependent activation of NF-κB. J Immunol 172:2522–2529
Assaraf YG (2006) The role of multidrug resistance efflux transporters in antifolate resistance and folate homeostasis. Drug Resist Updates 9:227–246
Attwa EM, Elkot RA, Abdelshafey AS, Hafez AR (2019) Subcutaneous methotrexate versus oral form for the treatment and prophylaxis of chronic plaque psoriasis. Dermatol Ther 32:e13051
Auboeuf D, Rieusset J, Fajas L, Vallier P, Frering V, Riou JP, Staels B, Auwerx J, Laville M, Vidal H (1997) Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator–activated receptors and liver X receptor-α in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes 46:1319–1327
Auten RL, Mason SN, Auten KM, Brahmajothi M (2009) Hyperoxia impairs postnatal alveolar epithelial development via NADPH oxidase in newborn mice. Am J Physiology-Lung Cell Mol Physiol 297:L134–L142
Badagnani I, Castro RA, Taylor TR, Brett CM, Huang CC, Stryke D, Kawamoto M, Johns SJ, Ferrin TE, Carlson EJ (2006) Interaction of methotrexate with organic-anion transporting polypeptide 1A2 and its genetic variants. J Pharmacol Exp Ther 318:521–529
Baeuerle PA, Baichwal VR (1997) NF-kB as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immunol 65:111–138
Bannwarth B, Labat L, Moride Y, Schaeverbeke T (1994) Methotrexate in rheumatoid arthritis: an update. Drugs 47:25–50
Bedoui Y, Guillot X, Sélambarom J, Guiraud P, Giry C, Jaffar-Bandjee MC, Ralandison S, Gasque P (2019) Methotrexate an old drug with new tricks. Int J Mol Sci 20:5023
Bello AE, Perkins EL, Jay R, Efthimiou P (2017) Recommendations for optimizing methotrexate treatment for patients with rheumatoid arthritis. Open Access Rheumatology: Research and Reviews 9:67–79
Berger J, Moller DE (2002) The mechanisms of action of PPARs. Annu Rev Med 53:409–435
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE (2014) Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 94:329–354
Bianchi G, Caporali R, Todoerti M, Mattana P (2016) Methotrexate and rheumatoid arthritis: current evidence regarding subcutaneous versus oral routes of administration. Adv Therapy 33:369–378
Braun J (2010) Optimal administration and dosage of methotrexate. Clin Exp Rheumatol 28:S46–51
Budzik GP, Colletti LM, Faltynek CR (2000) Effects of methotrexate on nucleotide pools in normal human T cells and the CEM T cell line. Life Sci 66:2297–2307
Caglayan C, Kandemir FM, Yildirim S, Kucukler S, Eser G (2019) Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level, oxidative stress, apoptosis and inflammation in rats. J Trace Elem Med Biol 54:69–78
Carrillo-Martinez EJ, Flores-Hernández FY, Salazar-Montes AM, Nario-Chaidez HF, Hernández-Ortega LD (2024) Quercetin, a flavonoid with great pharmacological capacity. Molecules 29:1000
Celik E, Oguzturk H, Sahin N, Turtay MG, Oguz F, Ciftci O (2016) Protective effects of hesperidin in experimental testicular ischemia/reperfusion injury in rats. Archives Med Sci 12:928–934
Çelik H, Kandemir FM, Caglayan C, Özdemir S, Çomaklı S, Kucukler S, Yardım A (2020) Neuroprotective effect of rutin against colistin-induced oxidative stress, inflammation and apoptosis in rat brain associated with the CREB/BDNF expressions. Mol Biol Rep 47:2023–2034
Çetin A, Çiftçi O, Otlu A (2016) Protective effect of hesperidin on oxidative and histological liver damage following carbon tetrachloride administration in Wistar rats. Archives Med Sci 12:486–493
Cha B, Lim JW, Kim H (2015) Jak1/Stat3 is an upstream signaling of NF-κB activation in Helicobacter pylori-induced IL-8 production in gastric epithelial AGS cells. Yonsei Med J 56:862–866
Chande N, Wang Y, MacDonald JK, McDonald JW (2014) Methotrexate for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews
Chen X-L, Kunsch C (2004) Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Design 10:879–891
Chen Z, Zhang Y, Ma L, Ni Y, Zhao H (2016) Nrf2 plays a pivotal role in protection against burn trauma-induced intestinal injury and death. Oncotarget 7:19272
Cheng Y, Chen M, Zhuang Q, Lin Bj C, Yy, Yang L, Liu Mb Q, Wc, Qiu Hq (2021) Genetic factors involved in delayed methotrexate elimination in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 68:e28858
Chi X, Yao W, Xia H, Jin Y, Li X, Cai J, Hei Z (2015) Elevation of HO-1 expression mitigates intestinal ischemia-reperfusion injury and restores tight junction function in a rat liver transplantation model. Oxidative Med Cell Longev 2015:986075
Choi Y-J, Kang J-S, Park JHY, Lee Y-J, Choi J-S, Kang Y-H (2003) Polyphenolic flavonoids differ in their antiapoptotic efficacy in hydrogen peroxide–treated human vascular endothelial cells. J Nutr 133:985–991
Choi SH, Suh GJ, Kwon WY, Kim KS, Park MJ, Kim T, Ko JI (2017) Apocynin suppressed the nuclear factor-κB pathway and attenuated lung injury in a rat hemorrhagic shock model. J Trauma Acute Care Surg 82:566–574
Chung SS, Kim M, Youn B-S, Lee NS, Park JW, Lee IK, Lee YS, Kim JB, Cho YM, Lee HK (2009) Glutathione peroxidase 3 mediates the antioxidant effect of peroxisome proliferator-activated receptor γ in human skeletal muscle cells. Mol Cell Biol 29:20–30
Cicero AF, Baggioni A (2016) Berberine and its role in chronic disease. Anti-inflammatory nutraceuticals and chronic diseases. Adv Exp Med Biol 928:27–45
Cipriani P, Ruscitti P, Carubbi F, Liakouli V, Giacomelli R (2014) Methotrexate: an old new drug in autoimmune disease. Expert Rev Clin Immunol 10:1519–1530
Coates LC, Merola JF, Grieb SM, Mease PJ, Duffin KC (2020) Methotrexate in psoriasis and psoriatic arthritis. J Rheumatol Supplement 96:31–35
Cruz LF, de Figueiredo GF, Pedro LP, Amorin YM, Andrade JT, Passos TF, Rodrigues FF, Souza ILA, Gonçalves TPR, dos Santos Lima LAR (2020) Umbelliferone (7-hydroxycoumarin): a non-toxic antidiarrheal and antiulcerogenic coumarin. Biomed Pharmacother 129:110432
Dahlgren D, Sjöblom M, Hellström PM, Lennernäs H (2021) Chemotherapeutics-induced intestinal mucositis: pathophysiology and potential treatment strategies. Front Pharmacol 12:681417
Datta A, Kim GA, Taylor JM, Gugino SF, Farrow KN, Schumacker PT, Berkelhamer SK (2015) Mouse lung development and NOX1 induction during hyperoxia are developmentally regulated and mitochondrial ROS dependent. Am J Physiology-Lung Cell Mol Physiol 309:L369–L377
de Araujo Junior RF, da Silva Reinaldo MPO, de Castro Brito GA, de Cavalcanti Franca P, de Moura Freire MA, de Medeiros CAX, de Araujo AA (2014) Olmesartan decreased levels of IL-1β and TNF-α, down-regulated MMP-2, MMP-9, COX-2, RANK/RANKL and up-regulated SOCs-1 in an intestinal mucositis model. PLoS One 10(3):e0120057
Deng Y, Tang K, Chen R, Nie H, Liang S, Zhang J, Zhang Y, Yang Q (2019) Berberine attenuates hepatic oxidative stress in rats with non–alcoholic fatty liver disease via the Nrf2/ARE signalling pathway. Experimental Therapeutic Med 17:2091–2098
Desmoulin SK, Hou Z, Gangjee A, Matherly LH (2012) The human proton-coupled folate transporter: Biology and therapeutic applications to cancer. Cancer Biol Ther 13:1355–1373
DiNicolantonio JJ, McCarty MF, O’Keefe JH (2022) Nutraceutical activation of Sirt1: a review. Open Heart 9:e002171
Dogra S, Singh N, Kumar S, Narang T, Handa S (2022) Comparison of overall efficacy and safety of oral versus subcutaneous methotrexate in severe psoriasis. Dermatol Ther 35:e15656
Drishya S, Dhanisha SS, Guruvayoorappan C (2022) Antioxidant-rich fraction of Amomum subulatum fruits mitigates experimental methotrexate‐induced oxidative stress by regulating TNF‐α, IL‐1β, and IL‐6 proinflammatory cytokines. J Food Biochem 46:e13855
El AE-DE-S, Sokar SS, Shebl AM, Mohamed DZ (2017) Antifibrotic effect of diethylcarbamazine combined with hesperidin against ethanol induced liver fibrosis in rats. Biomed Pharmacother 89:1196–1206
El Morsy E, Ahmed M (2020) Protective effects of lycopene on hippocampal neurotoxicity and memory impairment induced by bisphenol A in rats. Hum Exp Toxicol 39:1066–1078
El-Baz AM, Khodir AE, El-Sokkary MMA, Shata A (2020) The protective effect of Lactobacillus versus 5-aminosalicylic acid in ulcerative colitis model by modulation of gut microbiota and Nrf2/Ho-1 pathway. Life Sci 256:117927
El-Sheikh AA, Morsy MA, Hamouda AH (2016) Protective mechanisms of thymoquinone on methotrexate-induced intestinal toxicity in rats. Pharmacognosy Magazine 12:S76
Elsayed HRH, Anbar HS, Rabei MR, Adel M, El-Gamal R (2021) Eicosapentaenoic and docosahexaenoic acids attenuate methotrexate-induced apoptosis and suppression of splenic T, B-Lymphocytes and macrophages with modulation of expression of CD3, CD20 and CD68. Tissue Cell 72:101533
Elshazly SM, Elhassanny AE, Mahmoud NM (2020) Cilostazol protects against acetic acid-induced colitis in rats: possible role for cAMP/SIRT1 pathway. Eur J Pharmacol 881:173234
Elsherbiny NM, Zaitone SA, Mohammad HM, El-Sherbiny M (2018) Renoprotective effect of nifuroxazide in diabetes-induced nephropathy: impact on NFκB, oxidative stress, and apoptosis. Toxicol Mech Methods 28:467–473
Ezhilarasan D (2021) Hepatotoxic potentials of methotrexate: understanding the possible toxicological molecular mechanisms. Toxicology 458:152840
Feagan BG, Rochon J, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Gillies R, Hopkins M (1995) Methotrexate for the treatment of Crohn’s disease. N Engl J Med 332:292–297
Feuerstein JD, Ho EY, Shmidt E, Singh H, Falck-Ytter Y, Sultan S, Terdiman JP, Sultan S, Cohen BL, Chachu K (2021) AGA clinical practice guidelines on the medical management of moderate to severe luminal and perianal fistulizing Crohn’s disease. Gastroenterology 160:2496–2508
Firat O, Makay O, Yeniay L, Gokce G, Yenisey C, Coker A (2017) Omega-3 fatty acids inhibit oxidative stress in a rat model of liver regeneration. Annals Surg Treat Res 93:1–10
Fouad AA, Abdel-Aziz AM, Hamouda AA (2020) Diacerein downregulates NLRP3/Caspase-1/IL-1β and IL-6/STAT3 pathways of inflammation and apoptosis in a rat model of cadmium testicular toxicity. Biol Trace Elem Res 195:499–505
Friedman B, Cronstein B (2019) Methotrexate mechanism in treatment of rheumatoid arthritis. Joint bone Spine 86:301–307
Fu Y, Chung F-L (2018) Oxidative stress and hepatocarcinogenesis. Hepatoma Res 4:39
Gao T, Wang T, Wang Z, Cao J, Dong Y, Chen Y (2021) Melatonin-mediated MT2 attenuates colitis induced by dextran sodium sulfate via PI3K/AKT/Nrf2/SIRT1/RORα/NF-κB signaling pathways. Int Immunopharmacol 96:107779
Gautam R, Singh M, Gautam S, Rawat JK, Saraf SA, Kaithwas G (2016) Rutin attenuates intestinal toxicity induced by Methotrexate linked with anti-oxidative and anti-inflammatory effects. BMC Complement Altern Med 16:1–6
Germolec DR, Shipkowski KA, Frawley RP, Evans E (2018) Markers of inflammation. Immunotoxicity Testing: Methods and Protocols 1803:57–79
Giletti A, Esperon P (2018) Genetic markers in methotrexate treatments. Pharmacogenomics J 18:689–703
Girnun GD, Domann FE, Moore SA, Robbins ME (2002) Identification of a functional peroxisome proliferator-activated receptor response element in the rat catalase promoter. Mol Endocrinol 16:2793–2801
Gohar A (2019) Response to’Reply to Gohar on lungs, methotrexate and psoriasis, a comment on fatal, incidental, idiopathic pulmonary fibrosis in a patient receiving long-term low-dose methotrexate for psoriasis’. Clin Exp Dermatol 44:948–948
Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T (2017) 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohn’s Colitis 11:3–25
Grim J, Chládek J, Martínková J (2003) Pharmacokinetics and pharmacodynamics of methotrexate in non-neoplastic diseases. Clin Pharmacokinet 42:139–151
Grönroos M, Chen M, Jahnukainen T, Capitanio A, Aizman RI, Celsi G (2006) Methotrexate induces cell swelling and necrosis in renal tubular cells. Pediatr Blood Cancer 46:624–629
Grygiel-Górniak B (2014) Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications-a review. Nutr J 13:1–10
Guichard N, Guillarme D, Bonnabry P, Fleury-Souverain S (2017) Antineoplastic drugs and their analysis: a state of the art review. Analyst 142:2273–2321
Haase H, Overbeck S, Rink L (2008) Zinc supplementation for the treatment or prevention of disease: current status and future perspectives. Exp Gerontol 43:394–408
Hacker H, Karin M (2006) Regulation and function of IKK and IKK-related kinases. Sci STKE 2006(357):re13
Hafez MM, Al-Harbi NO, Al-Hoshani AR, Al-Hosaini KA, Al Shrari SD, Al Rejaie SS, Sayed-Ahmed MM, Al-Shabanah OA (2015) Hepato-protective effect of rutin via IL-6/STAT3 pathway in CCl 4-induced hepatotoxicity in rats. Biol Res 48:1–10
Hagos Y, Wolff NA (2010) Assessment of the role of renal organic anion transporters in drug-induced nephrotoxicity. Toxins 2:2055–2082
Hamed KM, Dighriri IM, Baomar AF, Alharthy BT, Alenazi FE, Alali GH, Alenazy RH, Alhumaidi NT, Alhulayfi DH, Alotaibi YB, Alhumaidan SS, Alhaddad ZA, Humadi AA, Alzahrani SA, Alobaid RH (2022) Overview of methotrexate toxicity: a comprehensive literature review. Cureus 14(9):e29518
Han E, Yun Y, Kim G, Lee Y-h, Wang HJ, Lee B-W, Cha BS, Kim BS, Kang ES (2016a) Effects of omega-3 fatty acid supplementation on diabetic nephropathy progression in patients with diabetes and hypertriglyceridemia. PLoS ONE 11:e0154683
Han X, Yao W, Liu Z, Li H, Zhang Z-J, Hei Z, Xia Z (2016b) Lipoxin A4 preconditioning attenuates intestinal ischemia reperfusion injury through Keap1/Nrf2 pathway in a lipoxin A4 receptor independent manner. Oxidative Med Cell Longev 2016:9303606
Hasan H, Bhushan S, Fijak M, Meinhardt A (2022) Mechanism of inflammatory associated impairment of sperm function, spermatogenesis and steroidogenesis. Front Endocrinol 13:897029
Hassan NM, Said E, Shehatou GS (2021) Nifuroxazide suppresses UUO-induced renal fibrosis in rats via inhibiting STAT-3/NF-κB signaling, oxidative stress and inflammation. Life Sci 272:119241
Hassanein EH, Shalkami A-GS, Khalaf MM, Mohamed WR, Hemeida RA (2019) The impact of Keap1/Nrf2, P38MAPK/NF-κB and Bax/Bcl2/caspase-3 signaling pathways in the protective effects of berberine against methotrexate-induced nephrotoxicity. Biomed Pharmacother 109:47–56
Hassanein EH, Kamel EO, Ali FE, Ahmed MA-R (2021) Berberine and/or zinc protect against methotrexate-induced intestinal damage: role of GSK-3β/NRF2 and JAK1/STAT-3 signaling pathways. Life Sci 281:119754
Hassanein EH, Althagafy HS, Atwa AM, Kozman MR, El-Sayed MIK, Soubh AA (2022) Taurine attenuated methotrexate-induced intestinal injury by regulating NF-κB/iNOS and Keap1/Nrf2/HO-1 signals. Life Sci 311:121180
Hassanein EH, Ali FE, Sayed MM, Mahmoud AR, Jaber FA, Kotob MH, Abd-Elhamid TH (2023a) Umbelliferone potentiates intestinal protective effect of Lactobacillus Acidophilus against methotrexate-induced intestinal injury: biochemical and histological study. Tissue Cell 82:102103
Hassanein EHM, Mohamed WR, Hussein RM, Arafa EA (2023b) Edaravone alleviates methotrexate-induced testicular injury in rats: implications on inflammation, steroidogenesis, and Akt/p53 signaling. Int Immunopharmacol 117:109969
Hatada EN, Krappmann D, Scheidereit C (2000) NF-κB and the innate immune response. Curr Opin Immunol 12:52–58
He Q, Zhou W, Xiong C, Tan G, Chen M (2015) Lycopene attenuates inflammation and apoptosis in post–myocardial infarction remodeling by inhibiting the nuclear factor–κB signaling pathway. Mol Med Rep 11:374–378
Herman RA, Veng-Pedersen P, Hoffman J, Koehnke R, Furst DE (1989) Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci 78:165–171
Hevener AL, Olefsky JM, Reichart D, Nguyen MA, Bandyopadyhay G, Leung H-Y, Watt MJ, Benner C, Febbraio MA, Nguyen A-K (2007) Macrophage PPARγ is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Investig 117:1658–1669
Hillson JL, Furst DE (1997) Pharmacology and pharmacokinetics of methotrexate in rheumatic disease: practical issues in treatment and design. Rheumatic Disease Clin North Am 23:757–778
Hoekstra M, Haagsma C, Neef C, Proost J, Knuif A, van de Laar M (2004) Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rhuematol 31:645–648
Hoque M, Akram T, Saha SN (2023) A review on Methotrexate used in rheumatoid arthritis. Int J Res 10:321–341
Hoult J, Payá M (1996) Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential. Gen Pharmacology: Vascular Syst 27:713–722
Howard SC, McCormick J, Pui C-H, Buddington RK, Harvey RD (2016) Preventing and managing toxicities of high-dose methotrexate. Oncologist 21:1471–1482
Huang C-S, Hu M-L (2011) Lycopene inhibits DNA damage and reduces hMTH1 mRNA expression in the liver of Mongolian gerbils treated with ferric nitrilotriacetate. Food Chem Toxicol 49:1381–1386
Huang G, Yuan M, Zhang J, Li J, Gong D, Li Y, Lin P, Huang L (2016) IL-6 mediates differentiation disorder during spermatogenesis in obesity-associated inflammation by affecting the expression of Zfp637 through the SOCS3/STAT3 pathway. Sci Rep 6:1–11
Huang X, Fang Q, Rao T, Zhou L, Zeng X, Tan Z, Chen L, Ouyang D (2020) Leucovorin ameliorated methotrexate induced intestinal toxicity via modulation of the gut microbiota. Toxicol Appl Pharmcol 391:114900
Ibrahim IM, Althagafy HS, Abd-Alhameed EK, Al-Thubiani WS, Hassanein EHM (2022) Promising hepatoprotective effects of lycopene in different liver diseases. Life Sci 310:121131
Impellizzeri D, Esposito E, Mazzon E, Paterniti I, Di Paola R, Bramanti P, Cuzzocrea S (2011) Effect of apocynin, a NADPH oxidase inhibitor, on acute lung inflammation. Biochem Pharmacol 81:636–648
Inoue K, Yuasa H (2014) Molecular basis for pharmacokinetics and pharmacodynamics of methotrexate in rheumatoid arthritis therapy. Drug Metab Pharmacokinet 29:12–19
Ishola I, Awogbindin I, Olubodun-Obadun T, Olajiga A, Adeyemi O (2023) Vinpocetine prevents rotenone-induced Parkinson disease motor and non-motor symptoms through attenuation of oxidative stress, neuroinflammation and α-synuclein expressions in rats. Neurotoxicology 96:37–52
Iskender H, Dokumacioglu E, Sen TM, Ince I, Kanbay Y, Saral S (2017) The effect of hesperidin and quercetin on oxidative stress, NF-κB and SIRT1 levels in a STZ-induced experimental diabetes model. Biomed Pharmacother 90:500–508
Jagadeesh GS, Nagoor Meeran M, Selvaraj P (2016) Protective effects of 7-hydroxycoumarin on dyslipidemia and cardiac hypertrophy in isoproterenol‐induced myocardial infarction in rats. J Biochem Mol Toxicol 30:120–127
Jahovic N, Şener G, Çevik H, Ersoy Y, Arbak S, Yeğen BÇ (2004) Amelioration of methotrexate-induced enteritis by melatonin in rats. Cell Biochem Function: Cell Biochem its Modulation Act Agents or Disease 22:169–178
Jakaria M, Azam S, Haque ME, Jo S-H, Uddin MS, Kim I-S, Choi D-K (2019) Taurine and its analogs in neurological disorders: focus on therapeutic potential and molecular mechanisms. Redox Biol 24:101223
Janbaz KH, Saeed SA, Gilani AH (2002) Protective effect of rutin on Paracetamol-and CCl4-induced hepatotoxicity in rodents. Fitoterapia 73:557–563
Jian YP, Yang G, Zhang LH, Liang JY, Zhou HL, Wang YS, Xu ZX (2022) Lactobacillus plantarum alleviates irradiation-induced intestinal injury by activation of FXR‐FGF15 signaling in intestinal epithelia. J Cell Physiol 237:1845–1856
Joerger M, Huitema A, Illerhaus G, Ferreri A (2012) Rational administration schedule for high-dose methotrexate in patients with primary central nervous system lymphoma. Leuk Lymphoma 53:1867–1875
Jundt J, Browne B, Fiocco G, Steele A, Mock D (1993) A comparison of low dose methotrexate bioavailability: oral solution, oral tablet, subcutaneous and intramuscular dosing. J Rhuematol 20:1845–1849
Kamalakkannan N, Prince PSM (2006) Rutin improves the antioxidant status in streptozotocin-induced diabetic rat tissues. Mol Cell Biochem 293:211–219
Kamel MY, Ahmed SM, Abdelzaher WY, Welson NN, Abdel-Aziz AM (2022) Role of IL-6/STAT3 pathway in mediating the protective effect of agomelatine against methotrexate-induced lung/intestinal tissues damage in rats. Immunopharmacol Immunotoxicol 44:35–46
Kandemir FM, Caglayan C, Aksu EH, Yildirim S, Kucukler S, Gur C, Eser G (2020) Protective effect of rutin on mercuric chloride-induced reproductive damage in male rats. Andrologia 52:e13524
Karageorgou D, Rova U, Christakopoulos P, Katapodis P, Matsakas L, Patel A (2023) Benefits of supplementation with microbial omega-3 fatty acids on human health and the current market scenario for fish-free omega-3 fatty acid. Trends in Food Science & Technology
Katturajan R, Evan Prince S (2023) Zinc and L-carnitine combination with or without methotrexate prevents intestinal toxicity during arthritis treatment via Nrf2/Sirt1/Foxo3 pathways: an in vivo and molecular docking approach. Inflammopharmacology 31:2599–2614
Kawaguchi S, Fujiwara S-i, Murahashi R, Nakashima H, Matsuoka S, Ikeda T, Toda Y, Ito S, Ban T, Nagayama T (2021) Risk factors for high-dose methotrexate-induced nephrotoxicity. Int J Hematol 114:79–84
Kesik V, Uysal B, Kurt B, Kismet E, Koseoglu V (2009) Ozone ameliorates methotrexate-induced intestinal injury in rats. Cancer Biol Ther 8:1623–1628
Khan ZA, Tripathi R, Mishra B (2012) Methotrexate: a detailed review on drug delivery and clinical aspects. Expert Opin Drug Deliv 9:151–169
Khodir AE, Said E (2020) Nifuroxazide attenuates experimentally-induced hepatic encephalopathy and the associated hyperammonemia and cJNK/caspase-8/TRAIL activation in rats. Life Sci 252:117610
Khongthong P, Roseweir AK, Edwards J (2019) The NF-KB pathway and endocrine therapy resistance in breast cancer. Endocrine-related Cancer 26:R369–R380
Khor TO, Huang M-T, Kwon KH, Chan JY, Reddy BS, Kong A-N (2006) Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium–induced colitis. Cancer Res 66:11580–11584
Kim Y-J, Song M, Ryu J-C (2009) Inflammation in methotrexate-induced pulmonary toxicity occurs via the p38 MAPK pathway. Toxicology 256:183–190
Kim S-K, Park K-Y, Yoon W-C, Park S-H, Park K-K, Yoo D-H, Choe J-Y (2011) Melittin enhances apoptosis through suppression of IL-6/sIL-6R complex-induced NF-κB and STAT3 activation and Bcl-2 expression for human fibroblast-like synoviocytes in rheumatoid arthritis. Joint Bone Spine 78:471–477
Kim SY, Moon KA, Jo HY, Jeong S, Seon SH, Jung E, Cho YS, Chun E, Lee KY (2012) Anti-inflammatory effects of apocynin, an inhibitor of NADPH oxidase, in airway inflammation. Immunol Cell Biol 90:441–448
Kirbas A, Cure MC, Kalkan Y, Cure E, Tumkaya L, Sahin OZ, Yuce S, Kizilkaya B, Pergel A (2015) Effect of infliximab on renal injury due to methotrexate in rat. Iran J Kidney Dis 9:221
Kobara M, Tatsumi T, Kambayashi D, Mano A, Yamanaka S, Shiraishi J, Keira N, Matoba S, Asayama J, Fushiki S (2003) Effects of ACE inhibition on myocardial apoptosis in an ischemia–reperfusion rat heart model. J Cardiovasc Pharmacol 41:880–889
Kobayashi A, Kang M-I, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M (2004) Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24:7130–7139
Kong Y, Olejar KJ, On SL, Chelikani V (2020) The potential of Lactobacillus spp. for modulating oxidative stress in the gastrointestinal tract. Antioxidants 9:610
Koppelmann T, Pollak Y, Ben-Shahar Y, Gorelik G, Sukhotnik I (2021) The mechanisms of the anti-inflammatory and anti-apoptotic effects of omega-3 polyunsaturated fatty acids during methotrexate-induced intestinal damage in cell line and in a rat model. Nutrients 13:888
Korbecki J, Bobiński R, Dutka M (2019) Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm Res 68:443–458
Korkmaz A, Kolankaya D (2013) Inhibiting inducible nitric oxide synthase with rutin reduces renal ischemia/reperfusion injury. Can J Surg 56:6
Kostakoglu U, Topcu A, Atak M, Tumkaya L, Mercantepe T, Uydu HA (2020) The protective effects of angiotensin-converting enzyme inhibitor against cecal ligation and puncture-induced sepsis via oxidative stress and inflammation. Life Sci 241:117051
Kremer J, Petrillo G, Hamilton R (1995) Pharmacokinetics and renal function in patients with rheumatoid arthritis receiving a standard dose of oral weekly methotrexate: association with significant decreases in creatinine clearance and renal clearance of the drug after 6 months of therapy. J Rhuematol 22:38–40
Kwon S, Seok S, Yau P, Li X, Kemper B, Kemper JK (2017) Obesity and aging diminish sirtuin 1 (SIRT1)-mediated deacetylation of SIRT3, leading to hyperacetylation and decreased activity and stability of SIRT3. J Biol Chem 292:17312–17323
Laurence A, Pesu M, Silvennoinen O, O’Shea J (2012) Suppl 2: JAK kinases in health and disease: an update. open Rheumatol J 6:232
Li Q, Verma IM (2002) NF-κB regulation in the immune system. Nat Rev Immunol 2:725–734
Li H-X, Zhao W, Shi Y, Li Y-N, Zhang L-S, Zhang H-Q, Wang D (2015) Retinoic acid amide inhibits JAK/STAT pathway in lung cancer which leads to apoptosis. Tumor Biology 36:8671–8678
Li J, Wang H, Zheng Z, Luo L, Wang P, Liu K, Namani A, Jiang Z, Wang XJ, Tang X (2018) Mkp-1 cross-talks with Nrf2/Ho-1 pathway protecting against intestinal inflammation. Free Radic Biol Med 124:541–549
Li CK, Baker K, Jones T, Coulson E, Roberts A, Birrell F (2021a) Safety and tolerability of subcutaneous methotrexate in routine clinical practice. Arthritis Care Res 73:1306–1311
Li J, Zhang Z, Wang L, Jiang L, Qin Z, Zhao Y, Su B (2021b) Maresin 1 attenuates lipopolysaccharide-induced acute kidney injury via inhibiting NOX4/ROS/NF-κB pathway. Front Pharmacol 12:782660
Li P, Inoue Y, Miyamoto D, Adachi T, Okada S, Adachi T, Soyama A, Hidaka M, Kanetaka K, Ito S (2023) Therapeutic effect and mechanism of Daikenchuto in a model of methotrexate-induced acute small intestinal mucositis. PLoS ONE 18:e0283626
Lin Y-C, Lin C-K, Tsai Y-H, Weng H-H, Li Y-C, You L, Chen J-K, Jablons DM, Yang C-T (2010) Adenovirus-mediated SOCS3 gene transfer inhibits the growth and enhances the radiosensitivity of human non-small cell lung cancer cells. Oncol Rep 24:1605–1612
Liu T, Zhang L, Joo D, Sun S-C (2017a) NF-κB signaling in inflammation. Signal Transduct Target Therapy 2:1–9
Liu Y, Bao Z, Xu X, Chao H, Lin C, Li Z, Liu Y, Wang X, You Y, Liu N (2017b) Extracellular signal-regulated kinase/nuclear factor-erythroid2-like2/heme oxygenase-1 pathway-mediated mitophagy alleviates traumatic brain injury-induced intestinal mucosa damage and epithelial barrier dysfunction. J Neurotrauma 34:2119–2131
Liu Y, Colby JK, Zuo X, Jaoude J, Wei D, Shureiqi I (2018) The role of PPAR-δ in metabolism, inflammation, and cancer: many characters of a critical transcription factor. Int J Mol Sci 19:3339
Logan RM, Stringer AM, Bowen JM, Yeoh AS-J, Gibson RJ, Sonis ST, Keefe DM (2007) The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal models and cytotoxic drugs. Cancer Treat Rev 33:448–460
Lopez-Gonzalez JS, Prado-Garcia H, Aguilar-Cazares D, Molina-Guarneros JA, Morales-Fuentes J, Mandoki JJ (2004) Apoptosis and cell cycle disturbances induced by coumarin and 7-hydroxycoumarin on human lung carcinoma cell lines. Lung Cancer 43:275–283
Lushchak VI (2014) Free radicals, reactive oxygen species, oxidative stress and its classification. Chemico-Biol Interact 224:164–175
Maksimovic V, Pavlovic-Popovic Z, Vukmirovic S, Cvejic J, Mooranian A, Al-Salami H, Mikov M, Golocorbin-Kon S (2020) Molecular mechanism of action and pharmacokinetic properties of methotrexate. Mol Biol Rep 47:4699–4708
Mansoury M, Almukadi H, Turkistani AM, Khattab HA, Ali SS, Hassanein EH, Alahmadi BA, Al-Jaouni S, El-Shitany NA (2023) Apocynin attenuates methotrexate-induced mucositis by regulating NF-κB, PPAR-γ and Bax/Bcl-2/Puma signals. Pak J Pharm Sci 36(2):457–466
Mao T, Han C, Wei B, Zhao L, Zhang Q, Deng R, Liu J, Luo Y, Zhang Y (2018) Protective effects of quercetin against cadmium chloride-induced oxidative injury in goat sperm and zygotes. Biol Trace Elem Res 185:344–355
Marra M, Sordelli IM, Lombardi A, Lamberti M, Tarantino L, Giudice A, Stiuso P, Abbruzzese A, Sperlongano R, Accardo M (2011) Molecular targets and oxidative stress biomarkers in hepatocellular carcinoma: an overview. J Translational Med 9:1–14
Marreiro DDN, Cruz KJC, Morais JBS, Beserra JB, Severo JS, De Oliveira ARS (2017) Zinc and oxidative stress: current mechanisms. Antioxidants 6:24
Mazimba O (2017) Umbelliferone: sources, chemistry and bioactivities review. Bull Fac Pharm Cairo Univ 55:223–232
Mei S, Li X, Jiang X, Yu K, Lin S, Zhao Z (2018) Population pharmacokinetics of high-dose methotrexate in patients with primary central nervous system lymphoma. J Pharm Sci 107:1454–1460
Meng X, Wei M, Wang D, Qu X, Zhang K, Zhang N, Li X (2020) The protective effect of hesperidin against renal ischemia-reperfusion injury involves the TLR-4/NF-κB/iNOS pathway in rats. Physiol Int 107:82–91
Mikhaylov D, Hashim PW, Nektalova T, Goldenberg G (2019) Systemic psoriasis therapies and comorbid disease in patients with psoriasis: a review of potential risks and benefits. J Clin Aesthetic Dermatol 12:46
Miyazono Y, Gao F, Horie T (2004) Oxidative stress contributes to methotrexate-induced small intestinal toxicity in rats. Scand J Gastroenterol 39:1119–1127
Mohamed YT, Naguib IA, Abo-Saif AA, Mohamed WR (2022) Protective effects of perindopril against indomethacin-induced gastric mucosal damage through modulation of DDAH-1/ADMA and ACE-2/ANG-(1–7) signaling pathways. Drug Chem Toxicol 45:2509–2518
Mohammed AS, Al-Hassani AN, Alrawi RA, Tawfeeq RD (2023) The protective effect of taurine, piracetam and vinpocetine on etoposide-induced inflammation and brain injury in the serum of female albino rats. Ecancermedicalscience 17:1499
Moreno S, Farioli-Vecchioli S, Ceru M (2004) Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience 123:131–145
Müller L, Fröhlich K, Böhm V (2011) Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem 129:139–148
Murakami T, Mori N (2012) Involvement of multiple transporters-mediated transports in mizoribine and methotrexate pharmacokinetics. Pharmaceuticals 5:802–836
Musa NS, Howarth GS, Tran CD (2015) Zinc supplementation alone is effective for partial amelioration of methotrexate-induced intestinal damage. Altern Ther Health Med 21:22–31
Nadeem RI, Ahmed HI, El-Sayeh BM (2018) Protective effect of vinpocetine against neurotoxicity of manganese in adult male rats. Naunyn Schmiedebergs Arch Pharmacol 391:729–742
Nafees S, Rashid S, Ali N, Hasan SK, Sultana S (2015) Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: role of NFκB/MAPK pathway. Chemico-Biol Interact 231:98–107
Natarajan K, Abraham P, Kota R, Isaac B (2018) NF-κB-iNOS-COX2-TNF α inflammatory signaling pathway plays an important role in methotrexate induced small intestinal injury in rats. Food Chem Toxicol 118:766–783
Navarro-García VM, Rojas G, Avilés M, Fuentes M, Zepeda G (2011) In vitro antifungal activity of coumarin extracted from Loeselia mexicana Brand. Mycoses 54:e569–e571
Neradil J, Pavlasova G, Veselská R (2012) New mechanisms for an old drug; DHFR-and non-DHFR-mediated effects of methotrexate in cancer cells. Klin Onkol 25(Suppl 2):2S87–2S92
Nicolas CS, Amici M, Bortolotto ZA, Doherty A, Csaba Z, Fafouri A, Dournaud P, Gressens P, Collingridge GL, Peineau S (2013) The role of JAK-STAT signaling within the CNS. Jak-stat 2:e22925
Nielsen OH, Steenholdt C, Juhl CB, Rogler G (2020) Efficacy and safety of methotrexate in the management of inflammatory bowel disease: a systematic review and meta-analysis of randomized, controlled trials. EClinicalMedicine 20:100271
Nowak A, Paliwoda A, Błasiak J (2019) Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: a review of mechanisms and therapeutic perspectives. Crit Rev Food Sci Nutr 59:3456–3467
Nuernberg B, Koehnke R, Solsky M, Hoffman J, Furst DE (1990) Biliary elimination of low-dose methotrexate in humans. Arthritis Rheumatism: Official J Am Coll Rheumatol 33:898–902
Nwokocha CR, Palacios J, Rattray VR, McCalla G, Nwokocha M, McGrowder D (2020) Protective effects of apocynin against cadmium toxicity and serum parameters; evidence of a cardio-protective influence. Inorg Chim Acta 503:119411
Oscarsson J, Hurt-Camejo E (2017) Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and their mechanisms of action on apolipoprotein B-containing lipoproteins in humans: a review. Lipids Health Dis 16:1–13
Ozcicek F, Kara AV, Akbas EM, Kurt N, Yazici GN, Cankaya M, Mammadov R, Ozcicek A, Suleyman H (2020) Effects of Anakinra on the small intestine mucositis induced by methotrexate in rats. Exp Anim 69:144–152
Pahwa R, Goyal A, Bansal P, Jialal I (2023) Chronic inflammation. In: StatPearls. StatPearls Publishing, Treasure Island (FL)
Palabiyik SS, Erkekoglu P, Zeybek ND, Kizilgun M, Baydar DE, Sahin G, Giray BK (2013) Protective effect of lycopene against ochratoxin A induced renal oxidative stress and apoptosis in rats. Exp Toxicol Pathol 65:853–861
Palkowitsch L, Leidner J, Ghosh S, Marienfeld RB (2008) Phosphorylation of serine 68 in the IκB kinase (IKK)-binding domain of NEMO interferes with the structure of the IKK complex and tumor necrosis factor-α-induced NF-κB activity. J Biol Chem 283:76–86
Palmquist D (2009) Omega-3 fatty acids in metabolism, health, and nutrition and for modified animal product foods. Prof Anim Sci 25:207–249
Park J, Chun J, Park SJ, Park JJ, Kim TI, Yoon H, Cheon JH (2023a) Effectiveness and tolerability of methotrexate monotherapy in Crohn’s disease patients: a multicenter observational study. Therapeutic Adv Gastroenterol 16:17562848231191664
Park NW, Lee ES, Ha KB, Jo SH, Kim HM, Kwon M-H, Chung CH (2023b) Umbelliferone ameliorates hepatic steatosis and lipid-Induced ER stress in High-Fat Diet-Induced obese mice. Yonsei Med J 64:243
Patel P, Shah J (2021) Protective effects of hesperidin through attenuation of Ki67 expression against DMBA-induced breast cancer in female rats. Life Sci 285:119957
Patyar S, Prakash A, Modi M, Medhi B (2011) Role of vinpocetine in cerebrovascular diseases. Pharmacol Rep 63:618–628
Pi D, Liu Y, Shi H, Li S, Odle J, Lin X, Zhu H, Chen F, Hou Y, Leng W (2014) Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J Nutr Biochem 25:456–462
Polat N, Ciftci O, Cetin A, Yılmaz T (2016) Toxic effects of systemic cisplatin on rat eyes and the protective effect of hesperidin against this toxicity. Cutan Ocul Toxicol 35:1–7
Rajitha P, Biswas R, Sabitha M, Jayakumar R (2017) Methotrexate in the treatment of psoriasis and rheumatoid arthritis: mechanistic insights, current issues and novel delivery approaches. Curr Pharm Design 23:3550–3566
Rao NA, Ambili M, Jala VR, Subramanya H, Savithri H (2003) Structure–function relationship in serine hydroxymethyltransferase. Biochim et Biophys Acta (BBA)-Proteins Proteom 1647:24–29
Rawlings JS, Rosler KM, Harrison DA (2004) The JAK/STAT signaling pathway. J Cell Sci 117:1281–1283
Reed DR, Pierce EJ, Sen JM, Keng MK (2019) A prospective study on urine alkalization with an oral regimen consisting of sodium bicarbonate and acetazolamide in patients receiving high-dose methotrexate. Cancer Manag Res 11:8065–8072
RFd AJ, da Silva Reinaldo M, GAdC B, PdF C, MAdM F (2014) Olmesartan decreased levels of IL-1b and TNF-a, down-regulated MMP-2, MMP-9, COX-2, RANK/RANKL and Up-Regulated SOCs-1 in an intestinal mucositis model. PLoS ONE 9:e114923
Ricote M, Huang JT, Welch JS, Glass CK (1999) The peroxisome proliferator-activated receptorγ (PPARγ) as a regulator of monocyte/macrophage function. J Leukoc Biol 66:733–739
Romao VC, Lima A, Bernardes M, Canhao H, Fonseca JE (2014) Three decades of low-dose methotrexate in rheumatoid arthritis: can we predict toxicity? Immunol Res 60:289–310
Ronalds BF (2019) Bring-ing together academic and industrial Chemistry: Edmund Ronalds’ Contri-Bution. Substantia 3:139–152
Rubino FM (2001) Separation methods for methotrexate, its structural analogues and metabolites. J Chromatogr B Biomed Sci Appl 764:217–254
Saini RK, Rengasamy KR, Mahomoodally FM, Keum Y-S (2020) Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: an update on epidemiological and mechanistic perspectives. Pharmacol Res 155:104730
Salminen A, Kaarniranta K, Kauppinen A (2013) Crosstalk between oxidative stress and SIRT1: impact on the aging process. Int J Mol Sci 14:3834–3859
Sami DH, Soliman AS, Khowailed AA, Hassanein EH, Kamel EM, Mahmoud AM (2022) 7-hydroxycoumarin modulates Nrf2/HO-1 and microRNA-34a/SIRT1 signaling and prevents cisplatin-induced oxidative stress, inflammation, and kidney injury in rats. Life Sci 310:121104
Sarker MT, Wang ZY, Yang H, Wan X, Emmanuel A (2021) Evaluation of the protective effect of lycopene on growth performance, intestinal morphology, and digestive enzyme activities of aflatoxinB1 challenged broilers. Anim Sci J 92:e13540
Sayed AM, Hassanein EHM, Ali FEM, Omar ZMM, Rashwan EK, Mohammedsaleh ZM, Abd El-Ghafar OAM (2021) Regulation of Keap-1/Nrf2/AKT and iNOS/NF-κB/TLR4 signals by apocynin abrogated methotrexate-induced testicular toxicity: mechanistic insights and computational pharmacological analysis. Life Sci 284:119911
Sayed AM, Abdel-Fattah MM, Arab HH, Mohamed WR, Hassanein EH (2022) Targeting inflammation and redox aberrations by perindopril attenuates methotrexate-induced intestinal injury in rats: role of TLR4/NF-κB and c-Fos/c-Jun pro-inflammatory pathways and PPAR-γ/SIRT1 cytoprotective signals. Chemico-Biol Interact 351:109732
Schaller L, Lauschke VM (2019) The genetic landscape of the human solute carrier (SLC) transporter superfamily. Hum Genet 138:1359–1377
Schiff MH, Jaffe JS, Freundlich B (2014) Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ≥ 15 mg may be overcome with subcutaneous administration. Ann Rheum Dis 73:1549–1551
Schofield RC, Ramanathan LV, Murata K, Grace M, Fleisher M, Pessin MS, Carlow DC (2015) Development and validation of a turbulent flow chromatography and tandem mass spectrometry method for the quantitation of methotrexate and its metabolites 7-hydroxy methotrexate and DAMPA in serum. J Chromatogr B 1002:169–175
Scorletti E, Byrne CD (2013) Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu Rev Nutr 33:231–248
Seideman P, Beck O, Eksborg S, Wennberg M (1993) The pharmacokinetics of methotrexate and its 7-hydroxy metabolite in patients with rheumatoid arthritis. Br J Clin Pharmacol 35:409–412
Semis HS, Kandemir FM, Kaynar O, Dogan T, Arikan SM (2021) The protective effects of hesperidin against paclitaxel-induced peripheral neuropathy in rats. Life Sci 287:120104
Şener G, Ekşioğlu-Demiralp E, Cetiner M, Ercan F, Şirvancı S, Gedik N, Yeğen B (2006) L-Carnitine ameliorates methotrexate-induced oxidative organ injury and inhibits leukocyte death. Cell Biol Toxicol 22:47–60
Shalkami A-GS, Hassan MI, Abd El-Ghany AA (2018) Perindopril regulates the inflammatory mediators, NF-κB/TNF-α/IL-6, and apoptosis in cisplatin-induced renal dysfunction. Naunyn Schmiedebergs Arch Pharmacol 391:1247–1255
Shalkami A-GS, Hassanein EH, Sayed AM, Mohamed WR, Khalaf MM, Hemeida RA (2021) Hepatoprotective effects of phytochemicals berberine and umbelliferone against methotrexate-induced hepatic intoxication: experimental studies and in silico evidence. Environ Sci Pollut Res 28:67593–67607
Shao D, Fry JL, Han J, Hou X, Pimentel DR, Matsui R, Cohen RA, Bachschmid MM (2014) A redox-resistant sirtuin-1 mutant protects against hepatic metabolic and oxidant stress. J Biol Chem 289:7293–7306
Sheikhbahaei F, Khazaei M, Rabzia A, Mansouri K, Ghanbari A (2016) Protective effects of thymoquinone against methotrexate-induced germ cell apoptosis in male mice. Int J Fertility Steril 9:541
Sherif IO, Al-Shaalan NH (2022) Hepatoprotective effect of Ginkgo biloba extract against methotrexate-induced hepatotoxicity via targeting STAT3/miRNA-21 axis. Drug Chem Toxicol 45:1723–1731
Shibayama Y, Ushinohama K, Ikeda R, Yoshikawa Y, Motoya T, Takeda Y, Yamada K (2006) Effect of methotrexate treatment on expression levels of multidrug resistance protein 2, breast cancer resistance protein and organic anion transporters Oat1, Oat2 and Oat3 in rats. Cancer Sci 97:1260–1266
Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E (2006) Dysfunctional KEAP1–NRF2 interaction in non-small-cell lung cancer. PLoS Med 3:e420
Singh RK, van Haandel L, Kiptoo P, Becker ML, Siahaan TJ, Funk RS (2019) Methotrexate disposition, anti-folate activity and efficacy in the collagen-induced arthritis mouse model. Eur J Pharmacol 853:264–274
Song D, Cheng Y, Li X, Wang F, Lu Z, Xiao X, Wang Y (2017) Biogenic nanoselenium particles effectively attenuate oxidative stress-induced intestinal epithelial barrier injury by activating the Nrf2 antioxidant pathway. ACS Appl Mater Interfaces 9:14724–14740
Sonis ST (2004) The pathobiology of mucositis. Nat Rev Cancer 4:277–284
Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber‐Durlacher J, Donnelly JP, Rubenstein EB (2004) Perspectives on cancer therapy‐induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer: Interdisciplinary Int J Am Cancer Soc 100:1995–2025
Stafeev I, Menshikov M, Tsokolaeva Z, Shestakova M, Parfyonova YV (2015) Molecular mechanisms of latent inflammation in metabolic syndrome. Possible role of sirtuins and peroxisome proliferator-activated receptor type γ. Biochem (Moscow) 80:1217–1226
Stefanska J, Pawliczak R (2008) Apocynin: molecular aptitudes. Mediat Inflamm 2008:106507
Stolk J, Hiltermann T, Dijkman J, Verhoeven A (1994) Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol 11:95–102
Sukhotnik I, Moati D, Shaoul R, Loberman B, Pollak Y, Schwartz B (2018) Quercetin prevents small intestinal damage and enhances intestinal recovery during methotrexate-induced intestinal mucositis of rats. Food Nutr Res 62:1327
Suzuki T, Motohashi H, Yamamoto M (2013) Toward clinical application of the Keap1–Nrf2 pathway. Trends Pharmacol Sci 34:340–346
Swanson D, Block R, Mousa SA (2012) Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr 3:1–7
Taguchi K, Motohashi H, Yamamoto M (2011) Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells 16:123–140
Tambağ AK, Kazak F, Peker Akalın P, Kutlu T (2021) The protective effect of rutin against methotrexate-induced nephrotoxicity in rats. Turk J Nephrol 30:218–223
Tan J, Wang J, Yang C, Zhu C, Guo G, Tang J, Shen H (2019) Antimicrobial characteristics of Berberine against prosthetic joint infection-related Staphylococcus aureus of different multi-locus sequence types. BMC Complement Altern Med 19:1–10
Tan W, Li Y, Chen M, Wang Y (2011) Berberine hydrochloride: anticancer activity and nanoparticulate delivery system. Int J Nanomedicine 6:1773–1777
Tang S, Leung J, Chan L, Eddy A, Lai K (2008) Angiotensin converting enzyme inhibitor but not angiotensin receptor blockade or statin ameliorates murine adriamycin nephropathy. Kidney Int 73:288–299
Tang D, Zeng T, Wang Y, Cui H, Wu J, Zou B, Tao Z, Zhang L, Garside GB, Tao S (2020) Dietary restriction increases protective gut bacteria to rescue lethal methotrexate-induced intestinal toxicity. Gut Microbes 12:1714401
Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y (2007) Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem 282:6823–6832
Tao R, Coleman MC, Pennington JD, Ozden O, Park S-H, Jiang H, Kim H-S, Flynn CR, Hill S, McDonald WH (2010) Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 40:893–904
Tashkandi HM, Althagafy HS, Jaber FA, Alamri T, Al-Abbas NS, Shaer NA, Harakeh S, Hassanein EH (2023) Vinpocetine mitigates methotrexate-induced duodenal intoxication by modulating NF-κB, JAK1/STAT-3, and RIPK1/RIPK3/MLKL signals. Immunopharmacol Immunotoxicol 46:11–19
Theiss AL, Vijay–Kumar M, Obertone TS, Jones DP, Hansen JM, Gewirtz AT, Merlin D, Sitaraman SV (2009) Prohibitin is a novel regulator of antioxidant response that attenuates colonic inflammation in mice. Gastroenterology 137:199–208 e196
Tian H, Cronstein BN (2007) Understanding the mechanisms of action of methotrexate. Bull NYU Hosp Jt Dis 65:168–173
Tornero Molina J, López Robledillo JC, Casamira Ruiz N (2021) Potential benefits of the self-administration of subcutaneous methotrexate with autoinjector devices for patients: a review. Drug, Healthcare and Patient Safety 13:81–94
Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, Adamina M, Armuzzi A, Bachmann O, Bager P (2020) ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohn’s Colitis 14:4–22
Tran CD, Howarth GS, Coyle P, Philcox JC, Rofe AM, Butler RN (2003) Dietary supplementation with zinc and a growth factor extract derived from bovine cheese whey improves methotrexate-damaged rat intestine. Am J Clin Nutr 77:1296–1303
Trushina E, McMurray C (2007) Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience 145:1233–1248
Tukukino C, Wallerstedt SM (2020) Drug information centre queries and responses about drug interactions over 10 years—A descriptive analysis. Basic Clin Pharmacol Toxicol 126:65–74
Tunalı-Akbay T, Sehirli O, Ercan F, Sener G (2010) Resveratrol protects against methotrexate-induced hepatic injury in rats. J Pharm Pharm Sci 13:303–310
Turk E, Kandemir FM, Yildirim S, Caglayan C, Kucukler S, Kuzu M (2019) Protective effect of hesperidin on sodium arsenite-induced nephrotoxicity and hepatotoxicity in rats. Biol Trace Elem Res 189:95–108
Unsal V, Belge-Kurutaş E (2017) Experimental hepatic carcinogenesis: oxidative stress and natural antioxidants. Open Access Macedonian J Med Sci 5:686
Urquhart BL, Gregor JC, Chande N, Knauer MJ, Tirona RG, Kim RB (2010) The human proton-coupled folate transporter (hPCFT): modulation of intestinal expression and function by drugs. Am J Physiology-Gastrointestinal Liver Physiol 298:G248–G254
van Meer L, Moerland M, Cohen AF, Burggraaf J (2014) Urinary kidney biomarkers for early detection of nephrotoxicity in clinical drug development. Br J Clin Pharmacol 77:947–957
Van Roon E, Van De Laar M (2010) Methotrexate bioavailability. Clin Experimental Rheumatology-Incl Supplements 28:S27
Vandewalle B, Moerman E, Lefebvre B, Defrance F, Gmyr V, Lukowiak B, Conte JK, Pattou F (2008) PPARγ-dependent and-independent effects of rosiglitazone on lipotoxic human pancreatic islets. Biochem Biophys Res Commun 366:1096–1101
VanWert AL, Sweet DH (2008) Impaired clearance of methotrexate in organic anion transporter 3 (Slc22a8) knockout mice: a gender specific impact of reduced folates. Pharm Res 25:453–462
Varin RM, Mulder P, Tamion F, Richard V, Henry J-P, Fo L, Lerebours G, Thuillez C (2000) Improvement of endothelial function by chronic angiotensin-converting enzyme inhibition in heart failure: role of nitric oxide, prostanoids, oxidant stress, and bradykinin. Circulation 102:351–356
Villarino AV, Kanno Y, O’Shea JJ (2017) Mechanisms and consequences of Jak–STAT signaling in the immune system. Nat Immunol 18:374–384
Visser K, van der Heijde D (2009) Optimal dosage and route of administration of methotrexate in rheumatoid arthritis: a systematic review of the literature. Ann Rheum Dis 68:1094–1099
Wahli W, Braissant O, Desvergne B (1995) Peroxisome proliferator activated receptors: transcriptional regulators of adipogenesis. Lipid Metabolism more… Chem Biology 2:261–266
Wanchao S, Chen M, Zhiguo S, Futang X, Mengmeng S (2018) Protective effect and mechanism of Lactobacillus on cerebral ischemia reperfusion injury in rats. Braz J Med Biol Res 51:e7172
Wang X-J, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT (2008) Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 29:1235–1243
Wang A, Yi X, Yu H, Dong B, Qiao S (2009) Free radical scavenging activity of Lactobacillus fermentum in vitro and its antioxidative effect on growing–finishing pigs. J Appl Microbiol 107:1140–1148
Wang S, Dougherty EJ, Danner RL (2016) PPARγ signaling and emerging opportunities for improved therapeutics. Pharmacol Res 111:76–85
Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D, Wang Y, Li W (2017) Antioxidant properties of probiotic bacteria. Nutrients 9:521
Wang J, Zhu H, Wang K, Yang Z, Liu Z (2020a) Protective effect of quercetin on rat testes against cadmium toxicity by alleviating oxidative stress and autophagy. Environ Sci Pollut Res 27:25278–25286
Wang Y, Cui S, Zheng J, Li Y, Li P, Hou H (2020b) Berberine ameliorates intestinal mucosal barrier dysfunction in nonalcoholic fatty liver disease (NAFLD) rats. J King Saud University-Science 32:2534–2539
Wang Z, Sun R, Wang G, Chen Z, Li Y, Zhao Y, Liu D, Zhao H, Zhang F, Yao J (2020c) SIRT3-mediated deacetylation of PRDX3 alleviates mitochondrial oxidative damage and apoptosis induced by intestinal ischemia/reperfusion injury. Redox Biol 28:101343
Warren RB, Mrowietz U, von Kiedrowski R, Niesmann J, Wilsmann-Theis D, Ghoreschi K, Zschocke I, Falk TM, Blödorn-Schlicht N, Reich K (2017) An intensified dosing schedule of subcutaneous methotrexate in patients with moderate to severe plaque-type psoriasis (METOP): a 52 week, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389:528–537
Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, Trentham DE (1985) Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med 312:818–822
Wellman AS, Metukuri MR, Kazgan N, Xu X, Xu Q, Ren NS, Czopik A, Shanahan MT, Kang A, Chen W (2017) Intestinal epithelial sirtuin 1 regulates intestinal inflammation during aging in mice by altering the intestinal microbiota. Gastroenterology 153:772–786
Wen Z, Liu W, Li X, Chen W, Liu Z, Wen J, Liu Z (2019) A protective role of the NRF2-Keap1 pathway in maintaining intestinal barrier function. Oxidative Med Cell Longev 2019:1759149
Whittle S, Hughes R (2004) Folate supplementation and methotrexate treatment in rheumatoid arthritis: a review. Rheumatology 43:267–271
Willson TM, Lambert MH, Kliewer SA (2001) Peroxisome proliferator–activated receptor γ and metabolic disease. Annu Rev Biochem 70:341–367
Xiao C, Hong H, Yu H, Yuan J, Guo C, Cao H, Li W (2018) MiR-340 affects gastric cancer cell proliferation, cycle, and apoptosis through regulating SOCS3/JAK-STAT signaling pathway. Immunopharmacol Immunotoxicol 40:278–283
Xin P, Xu X, Deng C, Liu S, Wang Y, Zhou X, Ma H, Wei D, Sun S (2020) The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharmacol 80:106210
Xu M, Wu S, Wang Y, Zhao Y, Wang X, Wei C, Liu X, Hao F, Hu C (2022) Association between high-dose methotrexate-induced toxicity and polymorphisms within methotrexate pathway genes in acute lymphoblastic leukemia. Front Pharmacol 13:1003812
Yamamoto Y, Gaynor RB (2001) Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J Clin Investig 107:135–142
Yang Y, Liu Z, Chen J, Wang X, Jiao Z, Wang Z (2024) Factors influencing methotrexate pharmacokinetics highlight the need for individualized dose adjustment: a systematic review. Eur J Clin Pharmacol 80:11–37
Yousra M, Elsherbiny NM, El-Shafey M, Said E (2020) The interplay of the inhibitory effect of nifuroxazide on NF-κB/STAT3 signaling attenuates acetic acid-induced ulcerative colitis in rats. Environ Toxicol Pharmacol 79:103433
Yucel Y, Tabur S, Gozeneli O, Kocarslan S, Seker A, Buyukaslan H, Şavik E, Aktumen A, Ozgonul A, Uzunkoy A (2016) The effects of lycopene on intestinal injury due to methotrexate in rats. Redox Rep 21:113–118
Yurtal Z, Altug ME, Unsaldi E, Secinti IE, Kucukgul A (2020) Investigation of neuroprotective and therapeutic effects of Hesperidin in experimental spinal cord Injury. Turkish Neurosurg 30:899–906
Zang Y-N, Si-Zheng W, Qin Y, Zhang J-R, Li-Bo Z, Xiao-Ling W (2019) Population pharmacokinetic study of delayed methotrexate excretion in children with acute lymphoblastic leukemia. Int J Clin Pharmacol Ther 57:402
Zhang L, Li J, Zong L, Chen X, Chen K, Jiang Z, Nan L, Li X, Li W, Shan T (2016) Reactive oxygen species and targeted therapy for pancreatic cancer. Oxidative Med Cell Longev 2016:1616781
Zhang Y-s, Li J-d, Yan C (2018) An update on vinpocetine: new discoveries and clinical implications. Eur J Pharmacol 819:30–34
Zhang J, Wang P, Xu F, Huang W, Ji Q, Han Y, Shao B, Li Y (2020) Protective effects of lycopene against AFB1-induced erythrocyte dysfunction and oxidative stress in mice. Res Vet Sci 129:103–108
Zhang H, Wang J, Lang W, Liu H, Zhang Z, Wu T, Li H, Bai L, Shi Q (2022) Albiflorin ameliorates inflammation and oxidative stress by regulating the NF-κB/NLRP3 pathway in Methotrexate-induced enteritis. Int Immunopharmacol 109:108824
Zhu L, Gu P, Shen H (2019) Protective effects of berberine hydrochloride on DSS-induced ulcerative colitis in rats. Int Immunopharmacol 68:242–251
Zorov DB, Juhaszova M, Sollott SJ (2006) Mitochondrial ROS-induced ROS release: an update and review. Biochim et Biophys Acta (BBA)-Bioenergetics 1757:509–517
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Gaber F. Ali, Emad H. M. Hassanein, Wafaa R. Mohamed: Investigation, design, reviewing and editing the manuscript. The authors confirm that no paper mill and artificial intelligence was used.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, G.F., Hassanein, E.H.M. & Mohamed, W.R. Molecular mechanisms underlying methotrexate-induced intestinal injury and protective strategies. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03164-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03164-x