Abstract
Asthma is a chronic pulmonary disease with marked infiltrating inflammatory cells and reduced respiratory performance. Echinochrome (Ech) is a dark-red pigment isolated from the sea urchin spines, shells, and ova. It has antioxidant, antimicrobial, and anti-inflammatory properties, but whether it can be used in asthma treatment has yet to be investigated. In this research, we aimed to study the inhibitory actions of Ech on allergic asthma symptoms in mice. Mice were divided into 4 groups (n = 8 for each): control, ovalbumin-challenged, and Ech-treated (0.1 and 1 mg/kg). At the end of the experiment, nasal scratching, lung oxidative stress, airway inflammation, and remodeling were assessed. In ovalbumin-challenged BALB/C mice, treatment with Ech significantly decreased nasal scratching, lung oxidative stress, inflammatory cell infiltration, mucus hyperproduction and hyperplasia of goblet cells, IgE levels, and inflammatory cytokines. It also inhibited NF-κB phosphorylation. This is the first study to investigate the immunomodulatory effect of Ech against allergic asthma in mice. According to our findings, we imply that Ech may be utilized as a treatment for allergic asthma.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asthma is a persistent airway condition in which immune dysregulation causes persistent inflammation, resulting in abnormal bronchoconstriction, mucus secretion, and respiratory problems in those suffering (Gillissen and Paparoupa 2015). Asthma is characterized by chronic airway inflammation caused by infiltrating eosinophils, T lymphocytes, and mast cells and releasing of pro-inflammatory cytokines and lipid mediators (Wang et al. 2021). More than 300 million individuals worldwide have asthma (Dharmage et al. 2019). According to the Global Initiative for Asthma (GINA), there might be 400 million cases of asthma by 2025 (Wang et al. 2022). The development of asthma has been associated with an imbalance between T helper (Th)1/Th2 immune responses (Zhu et al. 2020). Inappropriate, excessive Th2 response results in the increased release of Th2-related cytokines, such as interleukin(IL)-4, IL-5, and IL-13 (Zheng et al. 2019), which cause immunoglobulin E (IgE) synthesis, mucus hypersecretion, and accumulation of oxygen free radicals by the airway infiltrating eosinophils and other immune cells (Antunes et al. 2022). Allergic asthma is frequently associated with increased reactive oxygen species (ROS) formation and impaired antioxidant mechanisms, causing oxidative stress in the lungs (Zhu et al. 2021), which affects antioxidant activity, enhances the production of inflammatory mediators, and causes goblet cell hyperplasia (Malaquias et al. 2018).
The pathogenesis of asthma has been linked to several biochemical cascades, especially nuclear factor kappa beta (NF-κB) (Bai et al. 2022). NF-κB is a key mediator of immunological and inflammatory actions via stimulating target gene transcription and initiating inflammatory cytokines (Edwards et al. 2009; Zhou et al. 2014). The sustained activation of NF-κB is related to asthmatic inflammation. It has been shown that suppressing the NF-κB signaling cascade could ameliorate ovalbumin (OVA)-induced allergic asthma (Wang et al. 2018).
For the preclinical testing of asthma medications, researchers can choose from a variety of animal models of experimental asthma. OVA-induced experimental asthma is a common model for testing potential antiasthmatic drugs (Thakur et al. 2019). High levels of serum IgE, airway inflammation, epithelial hypertrophy, goblet cell hyperplasia, and airway hyperresponsiveness are reported in OVA-sensitized and challenged animals, making them a popular asthma model (Kim et al. 2019).
Corticosteroids and antileukotrienes are two principal anti-inflammatory medications for the treatment of asthma, which are successful for most patients (Ducharme 2004). Nevertheless, some people show serious asthma symptoms and resist such drugs (Woolcock 1993). Nowadays, discovering medications to treat and protect against severe asthmatic conditions is critical (Eger and Bel 2019).
Marine animals are one of the primary sources of new natural compounds with promising biological applications (Karthikeyan et al. 2022). Some marine natural products are of enormous importance because of their therapeutic significance (Papon et al. 2022). Echinochrome (Ech) is the most frequent naturally-occurring pigment found in sea urchin shells, spines, and ova, which has the most potent antioxidant properties (Mohamed 2021). Elimination of ROS, binding metal ions, and reducing lipid peroxidation are all among the antioxidant processes that Ech can use (Jeong et al. 2014). It has antioxidative, antiviral, antialgal, and antimicrobial properties (Park et al. 2019). A previous study has shown that Ech possesses antifibrotic and anti-inflammatory properties by suppressing fibroblast stimulation and proinflammatory cytokine expression (Park et al. 2021). For the first time, the current research is aimed at evaluating the effect of Ech on lung oxidative stress markers in addition to airway inflammation and remodeling.
Materials and methods
Chemicals and kits
Standard Ech (Vladivostok, Russia) and Dulbecco’s phosphate buffer saline (PBS) 10X were obtained from SEROX GmbH® (Mannheim, Germany). Ovalbumin, aluminum hydroxide, and other materials used in this research were obtained from Sigma-Aldrich (St. Louis, MO, USA). OVA-specific IgE (Cat No. E-20391Mo) and total IgE (Cat No. E-20550Mo) (Houston, TX, USA), IL-4 (Cat No. BMS613), IL-13 (Cat No. BMS6015), and IL-1β (Cat No. BMS6002) were obtained from Invitrogen by Thermo Fisher Scientific (USA). Rat anti-mouse PerCP-conjugated CD3+ antibody (Clone: 145-2C11) was from BD Biosciences (USA). Anti-mouse phospho-RELA (S536) polyclonal antibody (p-NFκB-p65) was from Cusabio, Biotech Co., Ltd. DAB-substrate kit was from Thermo Fisher Scientific (USA).
Animals
Female BALB/c mice (Mus musculus) (6–8 weeks old) were obtained from the National Research Center (Egypt). They were housed and grouped in sterile cages and fed, and had free access to water ad libitum. This study was approved by the Institutional Animal Care and Use Committee (IACUC) with a number of CU/I/F/32/22 at Cairo University in Egypt. All of the experimental procedures were carried out in accordance with international standards for the care and use of laboratory animals and performed in accordance with the advice provided in the most recent edition of the Guide for the Care and Use of Laboratory Animals, National Research Council, USA.
Ech isolation
The Amarowicz method was used to isolate the pigments in the shell and spines, with minor modifications (Amarowicz et al. 1994; Kuwahara et al. 2009). The shells and spines were cleaned in cold tepid water, dried in air for two days in the dark at 4°C, and ground after the internal organs were eliminated. The obtained powder was dissolved by the addition of 30 mL of 6 M HCl. With an equal volume of diethyl ether, the formed dark-red solution was separated four times. The obtained ether layer was treated with 5% NaCl. Then anhydrous sodium sulfate was added to remove water from the ether solution. Finally, the Heidolph rotary evaporator (Schwabach, Germany) evaporated the diethyl ether under reduced pressure. Ech was obtained and kept in the dark at −30°C.
High-performance liquid chromatography (HPLC) analysis
A Shimadzu HPLC system (Kyoto, Japan) was used, which included two LC20AD pumps, a DGU-20 A3 degasser, and an SPD-M20 A diode-array detector. With a 1.0 mL/min flow rate, chromatographic separation was performed using a Zorbax Eclipse Plus C18 column (250 mm 4.6 mm, 5 m, Agilent Acetonitrile/methanol (5:9), and 0.1% formic acid made up the binary mobile phase. An elution profile looked like this: 30–80% acetonitrile in formic acid for 0–25 min (linear gradient). The volume of injection was 20µL. Between 200 and 800 nm, the detection was noted. The data analysis system comprised the LC Solution (Shimadzu). DMSO was used to dissolve Ech at a concentration of 5 mg/mL.
OVA sensitization and inhalation
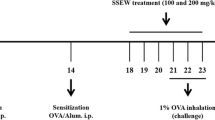
Following acclimatization for two weeks, 32 mice were randomly assigned into 4 groups (8 mice/group), including a control group, OVA group, low-dose Ech (0.1 mg/kg) group, and high-dose Ech (1 mg/kg) group. The asthma model was established according to Ou et al. (2021) with some modifications. The mice were sensitized intraperitoneally with 20 μg of OVA and 1 mg of alum gel dissolved in 200 μL of 0.9% saline on the 1st, 7th, and 14th days. From day 21 to 23, mice were placed into a container and challenged by atomization inhalation with a continuous dose of 2.5% OVA (1 hour per day). Mice from the control group were given the same volume of saline instead of OVA. One hour before challenging the mice, Ech was injected intraperitoneally for 7 days starting from day 17, while the control group was given the same amount of 2% DMSO, as illustrated in Fig. 1.
Sample collection
At the end of the experiment, by using isoflurane, mice were anesthetized and blood was drawn from the retro-orbital plexus for measuring the allergic and inflammatory mediators in the serum. The bronchoalveolar lavage fluid (BALF) was collected for identifying and quantifying the infiltrating inflammatory cells as well as the cytokine levels. Finally, the lung was obtained for the histopathological and immunohistochemical examination.
Evaluation of nasal scratching
Asthmatic behavior was evaluated according to Liu et al. (2022) with minor modifications. On the last day, nasal scratching was evaluated and scored for 10 min after the last challenge with 2.5% OVA. The scoring was as follows: mice who scratched their noses 0–2 times scored 0 points, 3–5 times scored 1 point, 6–8 times scored 2 points, and nine times or more scored 3 points.
Collection of BALF and cell count
The trachea was intubated and rinsed with 0.6 mL PBS buffer containing bovine serum albumin (BSA) and EDTA three times. Each mouse’s lavage solutions were preserved on ice and then centrifuged at 2000 rpm for 10 min at 4°C using a cooling centrifuge (Sigma, 3-30K, Germany); the supernatants were gathered and stored at −80 °C. The pellet was suspended in 100 μL PBS and stained with Wright–Giemsa stain. Total and differential inflammatory cell counts were calculated. By counting 100 cells per slide at a magnification of ×40, the slides were examined for differential cell count (Wang et al. 2019).
Measurement of OVA-specific IgE in serum and BALF
Blood was obtained from the retro-orbital plexus and then centrifuged at 3000 rpm for 15 min at 4°C to obtain serum, which was kept at −80°C. Serum and BALF levels of OVA-specific IgE were measured using ELISA kits by an ELISA plate reader (DAS Instruments, model A3, Rome, Italy).
Determination of serum total IgE, IL-4, and IL-1β and BALF IL-13 levels
Serum levels of total IgE, IL-4, and IL-1β were measured. At the same time, the BALF supernatant was used to measure the concentration of IL-13 using ELISA kits according to the manufacturer’s procedures.
Determination of lipid peroxidation, GSH level, and catalase and GST activities in the lung tissue
The supernatant was collected by centrifuging the lung tissues at 25000 rpm for 10 min at 4°C after weighting and homogenizing them in 0.1M Tris-HCl buffer (pH 7.4). Subsequently, the levels of malondialdehyde (MDA), nitric oxide (NO), glutathione (GSH), glutathione-S-transferase (GST), and catalase (CAT) activities were measured by commercial kits.
Lung histology
Mice were dissected and the lung was collected and fixed in 10% neutral buffer formalin for 24 hours; then, 4-µm-thick sections were cut and stained with hematoxylin and eosin (H&E) and periodic-acid Schiff (PAS). The extent of lung inflammation and hyperplasia of goblet cells was evaluated based on a previously reported scoring system (Tanaka et al. 2001). In brief, the semi-quantitative scoring system for lung inflammation was as follows: 0, no cells; 1, a few cells; 2, a ring of cells (1 cell layer deep); 3, a ring of cells (2–4 cells deep); and 4, a ring of cells (> 4 cells deep). At the same time, the system for mucus secretion was as follows: 0, < 0.5% PAS-positive cells; 1, < 25%; 2, 25–50%; 3, 50–75%; and 4, > 75%. Four different sections were used to score infiltrating immune cells and goblet cells.
Flow cytometric analysis
Fresh spleens were collected from each group of mice and a syringe piston was used to squeeze the samples in PBS gently. Cells were suspended in 1 mL of PBS and centrifuged for 10 min at 1300 rpm at 4°C. After discarding the supernatants and gently vortexing the pellets in 200 μL of PBS, 100 μL of suspended cells was put into all reaction tubes, then 3 μL rat anti-mouse PerCP-conjugated CD3+ antibody. After that, the reaction tubes were placed in the dark for 20 min, and 2 mL of the lysis buffer was added and placed again in the dark for 10 min. For 3 min, tubes were centrifuged. The pellet was rinsed with 1 mL of PBS supplemented with BSA and EDTA for washing after the supernatants were discarded and centrifuged. Finally, cell pellets were resuspended in 400 μL of PBS, and the supernatants were discarded before the analyses using FACS Melody (BD Biosciences, USA).
Immunohistochemistry (IHC)
Tissue sections were cut into 4-µm sections, de-paraffinized, rehydrated, and exposed to heat-induced antigen retrieval step for 15 min, followed by blocking steps for protein and endogenous peroxidases using BSA and hydrogen peroxide, respectively. After washing in PBS, tissue slides were incubated with anti-mouse phospho-RELA (S536) polyclonal antibody (p-NFκB-p65) (at a dilution of 1:200) for 12 h at the refrigerator; then HRP-labeled secondary antibody was applied. After washing, the DAB substrate was utilized to produce the color. Positive expression was assessed as area % using ImageJ software.
Statistical analysis
Statistical Package for the Social Sciences (SPSS) was utilized for statistical analyses (IBM). Mean ± SEM was used to express values. The differences between groups were evaluated using one-way analysis of variance (ANOVA). Graphs were drawn using the software of GraphPad Prism version 8. The analysis of flow cytometry data was performed using BD FACSDiva software version 6.1.1. Duncan’s post hoc test was employed to compare the group means, and P < 0.05 was regarded as statistically significant.
Results
HPLC data
As shown in Fig. 2, the HPLC analyses of isolated Ech revealed a significant peak with a retention period of 7.11 min that matched the standard Ech with a total concentration of 85.02%.
Ech attenuated nasal scratching and the infiltration of inflammatory cells in BALF
Shortness of breath, wheezing, and sneezing are common clinical signs of asthma. Although spotting these symptoms in rodents can be difficult, they show signs like scratching, tickling, and rapid breathing (Wang et al. 2015). It was noticed that OVA-challenged mice had significantly elevated overall nasal scratching scores. However, following Ech treatment, the scores significantly decreased, showing the ameliorative effect of Ech on asthma symptoms. Additionally, the inflammatory cell counts in the BALF of the OVA group were examined to study the impact of Ech on the infiltrating immune cells. The OVA group’s infiltrating inflammatory cells in BALF were notably greater than in the control group. Ech treatment (0.1 and 1 mg/kg) inhibited this infiltration in a dose-dependent way. These findings are shown in Table 1.
Ech decreased the serum and BALF levels of immunoglobulins and cytokines
The key characteristic of allergic asthma is a rise in blood IgE levels (Scirica et al. 2007). In asthmatic mice, the levels of IgE, OVA-specific IgE, IL-4, IL-1β, and IL-13 increased significantly (P <0.05). While Ech treatment dose-dependently decreased these mediators’ levels compared to the asthmatic group, as shown in Table 2.
Effect of Ech on oxidative stress parameters
Table 3 provides data on the lung oxidative stress markers (MDA, NO, GSH, CAT, and GST). It was found that OVA mice had significantly greater MDA and NO concentrations than the control group (P <0.05). In contrast, a significant decline (P <0.05) was shown in the Ech-treated group in a dose-dependent manner. Additionally, there was a significant decline (P <0.05) in the concentration of GSH and GST and CAT activities in asthmatic mice. Despite this, a significant rise (P <0.05) was noticed in the Ech-treated mice in a dose-dependent way.
Ech ameliorated pathological alterations in lung tissue
H&E and PAS were used to demonstrate airway infiltrating immune cells, goblet cell hyperplasia, and mucus secretion. The OVA group’s lung tissue displayed greater immune cell infiltration into the airways (Fig. 3), excessive mucus production, and goblet cell hyperplasia (Fig. 4). However, Ech treatment improved these changes in a dose-dependent way. The inflammation and mucus scores are shown in Table 4.
Effect of Ech on histopathological alterations in the lung tissue of mice of different groups, (H&E) (400X). a Control: the lung showed average bronchioles (B) with average epithelial lining and average blood vessels (BV). b OVA: the lung showed bronchioles with ulcerated epithelial lining, mildly congested blood vessels, thickened alveolar walls, and excessive peri-bronchiolar inflammatory infiltrate with scattered eosinophils. c 0.1 mg/kg Ech: bronchioles with average epithelial lining and increased peri-bronchiolar and peri-vascular inflammatory infiltrate and average blood vessels. d 1 mg/kg Ech: bronchioles with average epithelial lining, average blood vessels, average alveolar walls, and mild peri-bronchiolar and peri-vascular inflammatory infiltrate
Effect of Ech on the percentage of CD3+ cells
The percentage of CD3+ cells in asthmatic mice increased significantly (P <0.05) compared to the control group. However, a significant decline (P <0.05) was shown after Ech administration in a dose-dependent way (Fig. 5) (Table 5).
Immunohistochemical detection of phosphorylated-NF-κB p65
The immune expression of Phospho-NF-κB p65 is illustrated in Fig. 6. The lung p-NF-κB p65 area % of asthmatic mice increased significantly (P <0.05). However, a significant reduction (P < 0.05) was noticed after Ech treatment in a dose-dependent way (Table 6).
Discussion
Asthma is a persistent disorder of the bronchi. It is characterized by the accumulation of the airways by inflammatory cells and their secreted mediators, such as cytokine and chemokines, which cause inflammation, hyperreactivity, and irreversible airway blockage (Jung et al. 2008). Nowadays, corticosteroids are frequently used to manage asthma symptoms. However, their use is restricted due to resistance and severe problems. Additionally, this approach negatively impacts children’s bone mass and growth (Jassal 2015). Consequently, it is vital to investigate new potential therapeutic strategies as well as the underlying molecular pathophysiology of asthma. Animal models of OVA-induced airway inflammation share various cellular and molecular characteristics with human asthma (Aun et al. 2017). We examined the anti-inflammatory and antioxidant effects of Ech in an asthmatic OVA mouse model. Additionally, we evaluated behavioral, biochemical, histopathological, and immunohistopathological analyses. The effects of Ech on oxidative stress markers, antioxidant parameters, Th1 and Th2-related cytokines, IgE, infiltrating inflammatory cells, airway remodeling, and NF-κB activation were examined.
The pathophysiology of asthma was strongly influenced by Th2 cytokines such as IL-4, IL-5, and IL-13. These cytokines strongly correlate with inflammatory cell infiltration, IgE generation, eosinophil activation, and airway hyperresponsiveness (Ray and Cohn 1999). One of the most crucial cytokines for controlling Th2 inflammatory responses is IL-4, which stimulates the maturation of B cells and the switch to IgE (Renz et al. 1995). IL-13 is an essential immunomodulatory cytokine in the pathophysiology of bronchial inflammation. In terms of bronchial hyperresponsiveness and secretion of mucus, IL-13 is more significant than IL-4. Thus, inhibiting these Th2 cytokines may reduce allergic asthma (Barnes 2001). It was reported that Ech suppressed IL-4 and IL-13 in the atopic dermatitis model. In our investigation, mice treated with OVA had significantly greater levels of such cytokines, whereas Ech administration greatly reduced their production. Following OVA sensitization and inhalation, the serum and the BALF concentrations of OVA-specific IgE increased dramatically in the OVA group. At the same time, this rise was reduced in the Ech treatment groups. These outcomes are consistent with earlier research where OVA exposure raised IgE concentrations (Eftekhar et al. 2019). IL-1β induces the polarization of Th2, which activates infiltrating eosinophils and produces cytokines such as IL-5 (Rajizadeh et al. 2019). The concentration of IL-1β increased in the OVA-challenged group, while a significant decrease was shown after Ech administration. These results are consistent with previous results where the expression of IL-1β genes was significantly elevated in the OVA murine model of asthma (shakerinasab et al. 2022).
The development of asthma is highly affected by the infiltration of inflammatory cells (Chung 1986). Eosinophils and lymphocytes are among the inflammatory cells infiltrating the airways during OVA-induced asthma (Song et al. 2008). Our findings in this investigation showed that the infiltrating immune cells count was elevated in the OVA-challenged mice. In contrast, Ech treatment significantly reduced their infiltration. Although macrophage infiltration did not change, this could mean that Ech treatment influences the polarization of macrophage rather than the infiltration. These results can be supported by the study conducted by Oh et al. (2019) in which Ech administration stimulated the polarization of macrophages toward the M2 type, which helps to reduce inflammation and promote tissue repair. Additionally, flow cytometric analysis showed increased proliferation of CD3+ cells in the spleen of asthmatic mice. However, Ech treatment causes a notable decline in a dose-dependent manner.
Oxidative stress is a key mediator in the pathophysiology of asthma (Adam-Bonci et al. 2021). Previous studies showed that OVA-induced animals’ airways generate high ROS levels (Nishida et al. 2002). The accumulation of these ROS causes airway hyperresponsiveness (Sadeghi-Hashjin et al. 1996). MDA generation is one of the most critical determinants of oxidative stress. In this research, it was figured out that there was a significant rise in the MDA concentration in the OVA group. At the same time, a significant decrease was shown in the Ech-treated groups. These findings agree with the results investigated by Sadek et al. (2022), where the MDA level increased in septic rats, and Ech administration showed a significant decline in the MDA level.
NO is a potent free radical that combines with superoxide anions to generate peroxide nitrite, which can harm cell membranes (Malaquias et al. 2018). It may have a dual function: at normal concentrations, it is critical for signal transduction, and elevated NO and reactive nitrogen species levels can cause cell destruction (Yu et al. 2018). In our work, the lung tissue homogenate NO level was significantly elevated in the OVA-challenged mice. However, the Ech administration resulted in a statistically significant decline in NO level. Our findings are consistent with those made by Dweik et al. (2001), where the OVA challenge raised the level of NO in asthmatic airways.
GSH and CAT are essential antioxidants for alleviating lung cell fibrosis and damage in asthmatic patients (Rogers and Cismowski 2018). According to Martínez-Martos et al. (2014), GSH is a crucial biological antioxidant that inhibits the generation of free radicals. Generally, a reduction in GSH concentration reflects an elevation in ROS. In the study, GSH concentrations were decreased notably in the OVA group. At the same time, a significant rise was shown in the Ech treatment groups. Previous research indicated that OVA administration decreased GSH levels, which were then correlated to their deletion as a result of lipid peroxidation and ROS generation (Xiao et al. 2023). CAT can alleviate cell damage by converting H2O2 into water and oxygen (Sahiner et al. 2018). In this study, the activity of CAT was significantly reduced in OVA-challenged mice, while its activity was enhanced after Ech treatment. These results agree with the previous study (Dalouchi et al. 2021), where the CAT activity was reduced in the OVA-induced animal model. GSTs are essential in detoxifying various chemicals by combining the electrophilic molecules with GSH (Economopoulos and Sergentanis 2010). In this study, the activity of GST decreased significantly in asthmatic mice. However, a significant elevation in its activity was observed after Ech administration. These findings agree with a previous study where asthmatic mice’s lungs had reduced GST activity levels (Ajayi et al. 2022). In this research, we investigated the ameliorative effect of Ech against parameters associated with oxidative stress; this was achieved by observing changes in the production of MDA and NO as well as the levels of GSH, CAT, and GST. Our findings imply that Ech can efficiently attenuate oxidative stress and the harm it causes to the airways.
The lung histopathology results, which showed that OVA-challenged mice had higher scores for inflammation and mucus secretion than the control group, supported the biochemical findings. After Ech administration, these scores significantly decreased, and the histopathological changes were also attenuated. In another study, it was discovered that administering Ech reduced the level of oxidative stress caused by lipopolysaccharide in the lungs and inhibited airway lymphocyte infiltration (Kuznetsova et al. 2019). NF-κB plays a crucial role in the regulation of cytokine production (Huang et al. 2015). Previous research revealed that NF-κB activation contributed to airway inflammation in human and murine asthmatics (Bureau et al. 2000). The bronchial epithelium of asthmatic mice displayed a significant and rapid NF-κB p65 nuclear translocation (Zhang et al. 2015). It has been demonstrated that OVA-challenged mice had higher levels of phospho-NF-κB p65 (Zhang et al. 2017). The present immunohistochemical findings reveal that Ech significantly reduced the level of phospho-NF-кBp65. These results suggest that Ech helps treat asthma.
Conclusion
In conclusion, our findings as shown in Fig. 7 are the first to reveal that Ech effectively protects the lungs from oxidative stress and inflammation and that this is highly dependent on restoring the balance between ROS and antioxidants, improving airway remodeling, reducing mucus secretion and goblet cells hyperplasia, and suppressing Th2-related cytokines as well as IgE and OVA-specific IgE production. Moreover, Ech administration inhibited the phosphorylation of NF-κB P65. These findings imply that it may be an effective and helpful treatment for allergic asthma.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. Raw data will be made available on request.
References
Adam-Bonci T-I, Bonci E-A, Pârvu A-E, Herdean A-I, Moț A, Taulescu M, Ungur A, Pop R-M, Bocșan C, Irimie A (2021) Vitamin D supplementation: oxidative stress modulation in a mouse model of ovalbumin-induced acute asthmatic airway inflammation. Int J Mol Sci 22:7089. https://doi.org/10.3390/ijms22137089
Ajayi BO, Olajide TA, Olayinka ET (2022) 6-gingerol attenuates pulmonary inflammation and oxidative stress in mice model of house dust mite-induced asthma. Adv Redox Res 5:100036. https://doi.org/10.1016/j.arres.2022.100036
Amarowicz R, Synowiecki J, Shahidi F (1994) Sephadex LH-20 separation of pigments from shells of red sea urchin (Strongylocentrotus franciscanus). Food Chem 51:227–229. https://doi.org/10.1016/0308-8146(94)90262-3
Antunes GL, Silveira JS, Luft C, Greggio S, Venturin GT, Schmitz F, Biasibetti-Brendler H, Vuolo F, Dal-Pizzol F, da Costa JC, Wyse ATS, Pitrez PM, da Cunha AA (2022) Airway inflammation induces anxiety-like behavior through neuroinflammatory, neurochemical, and neurometabolic changes in an allergic asthma model. Metab Brain Dis 37:911–926. https://doi.org/10.1007/s11011-022-00907-8
Aun MV, Bonamichi-Santos R, Arantes-Costa FM, Kalil J, Giavina-Bianchi P (2017) Animal models of asthma: utility and limitations. J Asthma Allergy 10:293–301. https://doi.org/10.2147/JAA.S121092
Bai D, Sun T, Lu F, Shen Y, Zhang Y, Zhang B, Yu G, Li H, Hao J (2022) Eupatilin suppresses OVA-induced asthma by inhibiting NF-κB and MAPK and activating Nrf2 signaling pathways in mice. Int J Mol Sci 23:1582. https://doi.org/10.3390/ijms23031582
Barnes PJ (2001) Cytokine-directed therapies for asthma. J Allergy Clin Immunol 108:S72–S76. https://doi.org/10.1067/mai.2001.116435
Bureau F, Delhalle S, Bonizzi G, Fiévez L, Dogné S, Kirschvink N, Vanderplasschen A, Merville M-P, Bours V, Lekeux P (2000) Mechanisms of persistent NF-κB activity in the bronchi of an animal model of asthma. J Immunol 165:5822–5830. https://doi.org/10.4049/jimmunol.165.10.5822
Chung KF (1986) Role of inflammation in the hyperreactivity of the airways in asthma. Thorax 41:657–662. https://doi.org/10.1136/thx.41.9.657
Dalouchi F, Falak R, Bakhshesh M, Sharifiaghdam Z, Azizi Y, Aboutaleb N (2021) Human amniotic membrane mesenchymal stem cell-conditioned medium reduces inflammatory factors and fibrosis in ovalbumin-induced asthma in mice. Exp Physiol 106:544–554. https://doi.org/10.1113/EP088911
Dharmage SC, Perret JL, Custovic A (2019) Epidemiology of asthma in children and adults. Front Pediatr 7:246. https://doi.org/10.3389/fped.2019.00246
Ducharme FM (2004) Inhaled corticosteroids versus leukotriene antagonists as first-line therapy for asthma. Treat Respir Med 3:399–405. https://doi.org/10.2165/00151829-200403060-00006
Dweik RA, Comhair SAA, Gaston B, Thunnissen FBJM, Farver C, Thomassen MJ, Kavuru M, Hammel J, Abu-Soud HM, Erzurum SC (2001) NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci 98:2622–2627. https://doi.org/10.1073/pnas.051629498
Economopoulos KP, Sergentanis TN (2010) GSTM1, GSTT1, GSTP1, GSTA1 and colorectal cancer risk: a comprehensive meta-analysis. Eur J Cancer 46:1617–1631. https://doi.org/10.1016/j.ejca.2010.02.009
Edwards MR, Bartlett NW, Clarke D, Birrell M, Belvisi M, Johnston SL (2009) Targeting the NF-κB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol Ther 121:1–13. https://doi.org/10.1016/j.pharmthera.2008.09.003
Eftekhar N, Moghimi A, Mohammadian Roshan N, Saadat S, Boskabady MH (2019) Immunomodulatory and anti-inflammatory effects of hydro-ethanolic extract of Ocimum basilicum leaves and its effect on lung pathological changes in an ovalbumin-induced rat model of asthma. BMC Complement Altern Med 19:349. https://doi.org/10.1186/s12906-019-2765-4
Eger KA, Bel EH (2019) The emergence of new biologics for severe asthma. Curr Opin Pharmacol 46:108–115. https://doi.org/10.1016/j.coph.2019.05.005
Gillissen A, Paparoupa M (2015) Inflammation and infections in asthma. Clin Respir J 9:257–269. https://doi.org/10.1111/crj.12135
Huang C-S, Lin A-H, Yang T-C, Liu K-L, Chen H-W, Lii C-K (2015) Shikonin inhibits oxidized LDL-induced monocyte adhesion by suppressing NFκB activation via up-regulation of PI3K/Akt/Nrf2-dependent antioxidation in EA.hy926 endothelial cells. Biochem Pharmacol 93:352–361. https://doi.org/10.1016/j.bcp.2014.12.005
Jassal MS (2015) Special considerations—asthma in children. Int Forum Allergy Rhinol 5:S61–S67. https://doi.org/10.1002/alr.21577
Jeong SH, Kim HK, Song I-S, Noh SJ, Marquez J, Ko KS, Rhee BD, Kim N, Mishchenko NP, Fedoreyev SA, Stonik VA, Han J (2014) Echinochrome A increases mitochondrial mass and function by modulating mitochondrial biogenesis regulatory genes. Mar Drugs 12:4602–4615. https://doi.org/10.3390/md12084602
Jung W-K, Lee D-Y, Choi YH, Yea SS, Choi I, Park S-G, Seo S-K, Lee S-W, Lee C-M, Kim S-k, Jeon Y-J, Choi I-W (2008) Caffeic acid phenethyl ester attenuates allergic airway inflammation and hyperresponsiveness in murine model of ovalbumin-induced asthma. Life Sci 82:797–805. https://doi.org/10.1016/j.lfs.2008.01.014
Karthikeyan A, Joseph A, Nair BG (2022) Promising bioactive compounds from the marine environment and their potential effects on various diseases. J Genet Eng Biotechnol 20:14. https://doi.org/10.1186/s43141-021-00290-4
Kim DI, Song M-K, Lee K (2019) Comparison of asthma phenotypes in OVA-induced mice challenged via inhaled and intranasal routes. BMC Pulm Med 19:241. https://doi.org/10.1186/s12890-019-1001-9
Kuwahara R, Hatate H, Yuki T, Murata H, Tanaka R, Hama Y (2009) Antioxidant property of polyhydroxylated naphthoquinone pigments from shells of purple sea urchin Anthocidaris crassispina. LWT - Food Sci Technol 42:1296–1300. https://doi.org/10.1016/j.lwt.2009.02.020
Kuznetsova M, Lebed’Ko O, Ryzhavskii B, Mishchenko N (2019) Effect of oral administration of echinochrome on lipopolysaccharide-induced lung injury in the immature Wistar rats. Eur Respir J 54:2360. https://doi.org/10.1183/13993003.congress-2019.PA2360
Liu C, You J, Lu Y, Sun J, Pan J, Li Y, Liu T, Li Y, Wang A, Zhang X, Wang Y, Pan W (2022) Protective effects on ovalbumin-induced mouse asthma models and qualitative and quantitative analysis of multiple compounds in Gerberae Piloselloidis Herba. J Sep Sci 45:990–1005. https://doi.org/10.1002/jssc.202100392
Malaquias MAS, Oyama LA, Jericó PC, Costa I, Padilha G, Nagashima S, Lopes-Pacheco M, Rebelatto CLK, Michelotto PV, Xisto DG, Brofman PRS, Rocco PRM, de Noronha L (2018) Effects of mesenchymal stromal cells play a role the oxidant/antioxidant balance in a murine model of asthma. Allergol Immunopathol 46:136–143. https://doi.org/10.1016/j.aller.2017.06.003
Martínez-Martos JM, Mayas MD, Carrera P, Arias de Saavedra JM, Sánchez-Agesta R, Arrazola M, Ramírez-Expósito MJ (2014) Phenolic compounds oleuropein and hydroxytyrosol exert differential effects on glioma development via antioxidant defense systems. J Funct Foods 11:221–234. https://doi.org/10.1016/j.jff.2014.09.006
Mohamed AS (2021) Echinochrome exhibits antitumor activity against ehrlich ascites carcinoma in Swiss albino mice. Nutr Cancer 73:124–132. https://doi.org/10.1080/01635581.2020.1737152
Nishida S, Teramoto K, Kimoto-Kinoshita S, Tohda Y, Nakajima S, Tomura TT, Irimajiri K (2002) Change of Cu, Zn-superoxide dismutase activity of guinea pig lung in experimental asthma. Free Radic Res 36:601–606. https://doi.org/10.1080/10715760210872
Oh S-J, Seo Y, Ahn J-S, Shin YY, Yang JW, Kim HK, Han J, Mishchenko NP, Fedoreyev SA, Stonik VA, Kim H-S (2019) Echinochrome A reduces colitis in mice and induces in vitro generation of regulatory immune cells. Mar Drugs 17:622. https://doi.org/10.3390/md17110622
Ou G, Liu Q, Yu C, Chen X, Zhang W, Chen Y, Wang T, Luo Y, Jiang G, Zhu M, Li H, Zeng M (2021) The protective effects of Maresin 1 in the OVA-induced asthma mouse model. Mediators Inflamm 2021:4131420. https://doi.org/10.1155/2021/4131420
Papon N, Copp BR, Courdavault V (2022) Marine drugs: biology, pipelines, current and future prospects for production. Biotechnol Adv 54:107871. https://doi.org/10.1016/j.biotechadv.2021.107871
Park G-B, Kim M-J, Vasileva EA, Mishchenko NP, Fedoreyev SA, Stonik VA, Han J, Lee HS, Kim D, Jeong J-Y (2019) Echinochrome A promotes ex vivo expansion of peripheral blood-derived CD34+ cells, potentially through downregulation of ROS production and activation of the Src-Lyn-p110δ pathway. Mar Drugs 17:526. https://doi.org/10.3390/md17090526
Park G-T, Yoon J-W, Yoo S-B, Song Y-C, Song P, Kim H-K, Han J, Bae S-J, Ha K-T, Mishchenko NP, Fedoreyev SA, Stonik VA, Kim M-B, Kim J-H (2021) Echinochrome A treatment alleviates fibrosis and inflammation in bleomycin-induced scleroderma. Mar Drugs 19:237. https://doi.org/10.3390/md19050237
Rajizadeh MA, Najafipour H, Fekr MS, Rostamzadeh F, Jafari E, Bejeshk MA, Masoumi-Ardakani Y (2019) Anti-inflammatory and anti-oxidative effects of myrtenol in the rats with allergic asthma. Iran J Pharm Sci IJPR 18:1488. https://doi.org/10.22037/ijpr.2019.1100749
Ray A, Cohn L (1999) Th2 cells and GATA-3 in asthma: new insights into the regulation of airway inflammation. J Clin Investig 104:985–993. https://doi.org/10.1172/JCI8204
Renz H, Enssle K, Lauffer L, Kurrle R, Gelfand EW (1995) Inhibition of allergen-induced IgE and IgG1 production by soluble IL-4 receptor. Int Arch Allergy Immunol 106:46–54. https://doi.org/10.1159/000236889
Rogers LK, Cismowski MJ (2018) Oxidative stress in the lung – the essential paradox. Curr Opin Toxicol 7:37–43. https://doi.org/10.1016/j.cotox.2017.09.001
Sadeghi-Hashjin G, Folkerts G, Henricks PA, Verheyen AK, van der Linde HJ, van Ark I, Coene A, Nijkamp FP (1996) Peroxynitrite induces airway hyperresponsiveness in guinea pigs in vitro and in vivo. Am J Respir Crit Care Med 153:1697–1701. https://doi.org/10.1164/ajrccm.153.5.8630623
Sadek SA, Hassanein SS, Mohamed AS, Soliman AM, Fahmy SR (2022) Echinochrome pigment extracted from sea urchin suppress the bacterial activity, inflammation, nociception, and oxidative stress resulted in the inhibition of renal injury in septic rats. J Food Biochem 46:e13729. https://doi.org/10.1111/jfbc.13729
Sahiner UM, Birben E, Erzurum S, Sackesen C, Kalayci Ö (2018) Oxidative stress in asthma: part of the puzzle. Pediatr Allergy Immunol 29:789–800. https://doi.org/10.1111/pai.12965
Scirica CV, Gold DR, Ryan L, Abulkerim H, Celedón JC, Platts-Mills TAE, Naccara LM, Weiss ST, Litonjua AA (2007) Predictors of cord blood IgE levels in children at risk for asthma and atopy. J Allergy Clin Immunol 119:81–88. https://doi.org/10.1016/j.jaci.2006.09.002
shakerinasab N, Bejeshk MA, Pourghadamyari H, Najafipour H, Eftekhari M, Mottaghipisheh J, Omidifar N, Azizi M, Rajizadeh MA, Doustimotlagh AH (2022) The hydroalcoholic extract of nasturtium officinale reduces lung inflammation and oxidative stress in an ovalbumin-induced rat model of asthma. Evid-based Complement Altern Med 2022:5319237. https://doi.org/10.1155/2022/5319237
Song C, Luo L, Lei Z, Li B, Liang Z, Liu G, Li D, Zhang G, Huang B, Feng Z-H (2008) IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol 181:6117–6124. https://doi.org/10.4049/jimmunol.181.9.6117
Tanaka H, Masuda T, Tokuoka S, Komai M, Nagao K, Takahashi Y, Nagai H (2001) The effect of allergen-induced airway inflammation on airway remodeling in a murine model of allergic asthma. Inflamm Res 50:616–624. https://doi.org/10.1007/PL00000243
Thakur VR, Khuman V, Beladiya JV, Chaudagar KK, Mehta AA (2019) An experimental model of asthma in rats using ovalbumin and lipopolysaccharide allergens. Heliyon 5:e02864. https://doi.org/10.1016/j.heliyon.2019.e02864
Wang Z-w, Li R-k, Ren Y, Liu X-f, Cheng X-l, Tuo H-y (2015) Establishment and evaluation of a mouse model of bronchial asthma with Yin deficiency syndrome. Chin J Appl Physiol 31:556–560
Wang C, Choi YH, Xian Z, Zheng M, Piao H, Yan G (2018) Aloperine suppresses allergic airway inflammation through NF-κB, MAPK, and Nrf2/HO-1 signaling pathways in mice. Int Immunopharmacol 65:571–579. https://doi.org/10.1016/j.intimp.2018.11.003
Wang J, Diao X, Zhu H, He B (2019) Effect of tiotropium bromide on airway inflammation and programmed cell death 5 in a mouse model of ovalbumin-Induced Allergic Asthma. Can Respir J 2019:6462171. https://doi.org/10.1155/2019/6462171
Wang G, Zhou B, Wang Z, Meng Y, Liu Y, Yao X, Feng C (2021) Pharmacological mechanisms underlying the anti-asthmatic effects of modified guomin decoction determined by network pharmacology and molecular docking. Front Mol Biosci 8:644561. https://doi.org/10.3389/fmolb.2021.644561
Wang Y, Zhu H, Tong J, Li Z (2022) Ligustrazine inhibits lung phosphodiesterase activity in a rat model of allergic asthma. Comput Math Methods Med 2022:1452116. https://doi.org/10.1155/2022/1452116
Woolcock AJ (1993) Steroid resistant asthma: what is the clinical definition? Eur Respir J 6:743. https://doi.org/10.1183/09031936.93.06050743
Xiao S, Zhou Y, Gao H, Yang D (2023) Dexmedetomidine attenuates airway inflammation and oxidative stress in asthma via the Nrf2 signaling pathway. Mol Med Rep 27:2. https://doi.org/10.3892/mmr.2022.12889
Yu X, Ge L, Niu L, Lian X, Ma H, Pang L (2018) The dual role of inducible nitric oxide synthase in myocardial ischemia/reperfusion injury: friend or foe? Oxidative Med Cell Longev 2018:8364848. https://doi.org/10.1155/2018/8364848
Zhang T, Yang S, Du J, Jinfu Y, Shumin W (2015) Platycodin D attenuates airway inflammation in a mouse model of allergic asthma by regulation NF-κB pathway. Inflamm 38:1221–1228. https://doi.org/10.1007/s10753-014-0089-6
Zhang Q, Wang L, Chen B, Zhuo Q, Bao C, Lin L (2017) Propofol inhibits NF-κB activation to ameliorate airway inflammation in ovalbumin (OVA)-induced allergic asthma mice. Int Immunopharmacol 51:158–164. https://doi.org/10.1016/j.intimp.2017.08.015
Zheng M, Guo X, Pan R, Gao J, Zang B, Jin M (2019) Hydroxysafflor Yellow A Alleviates ovalbumin-induced asthma in a guinea pig model by attenuateing the expression of inflammatory cytokines and signal transduction. Front Pharmacol 10:328. https://doi.org/10.3389/fphar.2019.00328
Zhou E, Fu Y, Wei Z, Yang Z (2014) Inhibition of allergic airway inflammation through the blockage of NF-κB activation by ellagic acid in an ovalbumin-induced mouse asthma model. Food Funct 5:2106–2112. https://doi.org/10.1039/C4FO00384E
Zhu X, Cui J, Yi L, Qin J, Tulake W, Teng F, Tang W, Wei Y, Dong J (2020) The role of T cells and macrophages in asthma pathogenesis: a new perspective on mutual crosstalk. Mediators Inflamm 2020:7835284. https://doi.org/10.1155/2020/7835284
Zhu Y, Wang C, Luo J, Hua S, Li D, Peng L, Liu H, Song L (2021) The protective role of Zingerone in a murine asthma model via activation of the AMPK/Nrf2/HO-1 pathway. Food Funct 12:3120–3131. https://doi.org/10.1039/D0FO01583K
Acknowledgments
We thank Cairo University for facilitating the use of instruments.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Abeer M. Badr, Islam Ahmed Abdelmawgood, and Ayman Saber Mohamed. The first draft of the manuscript was written by Noha Mahana and Islam Ahmed Abdelmawgood. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Institutional Animal Care and Use Committee (IACUC) with number of CU/I/F/32/22) at Cairo University in Egypt. All of the experimental procedures were carried out in accordance with international standards for the care and use of laboratory animals and performed in accordance with the advice provided in the most recent edition of the Guide for the Care and Use of Laboratory Animals, National Research Council, USA.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelmawgood, I.A., Mahana, N.A., Badr, A.M. et al. Echinochrome exhibits anti-asthmatic activity through the suppression of airway inflammation, oxidative stress, and histopathological alterations in ovalbumin-induced asthma in BALB/c mice. Naunyn-Schmiedeberg's Arch Pharmacol 397, 1803–1815 (2024). https://doi.org/10.1007/s00210-023-02678-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02678-0