Abstract
Purpose
Lung fibrosis is a heterogeneous lung condition characterized by excessive accumulation of scarred tissue, leading to lung architecture destruction and restricted ventilation. The current work was conducted to examine the probable shielding influence of cinnamic acid against lung fibrosis induced by methotrexate.
Methods
Rats were pre-treated with oral administration of cinnamic acid (50 mg/kg/day) for 14 days, whereas methotrexate (14 mg/kg) was orally given on the 5th and 12th days of the experiment. Pirfenidone (50 mg/kg/day) was used as a standard drug. At the end of the experiment, oxidative parameters (malondialdehyde, myeloperoxidase, nitric oxide, and total glutathione) and inflammatory mediators (tumor necrosis factor-α and interleukin-8), as well as transforming growth factor-β and collagen content, as fibrosis indicators, were measured in lung tissue.
Results
Our results revealed that cinnamic acid, as pirfenidone, effectively prevented the methotrexate-induced overt histopathological damage. This was associated with parallel improvements in oxidative, inflammatory, and fibrotic parameters measured. The outcomes of cinnamic acid administration were more or less the same as those of pirfenidone. In conclusion, pre-treatment with cinnamic acid protects against methotrexate-induced fibrosis, making it a promising prophylactic adjuvant therapy to methotrexate and protecting against its possible induction of lung fibrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methotrexate (MTX) has been widely prescribed in the treatment of a variety of malignant and inflammatory diseases for more than six decades and is still used for its remarkable therapeutic effects (Olsen et al. 2014; Dong et al. 2021). Furthermore, MTX possesses immunomodulatory properties due to its antimetabolite action, which interferes with folic acid metabolism. As MTX has a larger affinity for dihydrofolate reductase (DHFR) than folate, it inhibits the production of tetrahydrofolate reductase, which is essential for the biosynthesis of thymidine and purines, both of which are required for DNA synthesis (Rana et al. 2020; Chen et al. 2021). That is how it prevents cell division and protein production (Howard et al. 2016; Ghoneum and El-Gerbed 2021).
The initial instance of MTX-induced lung toxicity was documented in 1969 in children diagnosed with acute lymphoblastic leukemia (Jani et al. 2017). A significant proportion (60–93%) of individuals taking MTX medication encountered a range of clinical indications, such as difficulty breathing, coughing, elevated body temperature, breathing difficulties, pneumonia, lung inflammation, and fibrosis. Consequently, approximately 30% of these patients were compelled to halt MTX treatment as a consequence of these adverse reactions (Mammadov et al. 2019). A suggested mechanism for the development of lung toxicity caused by MTX involves the stimulation of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukins (IL), and monocyte chemoattractant protein (MCP-1) release through the p38 mitogen-activated protein kinase (MAPK) pathway (Kalemci et al. 2018; Koppelmann et al. 2021). Excessive production of these cytokines, along with platelet-derived growth factor (PDGF) and transforming growth factor- β (TGF- β), leads to the proliferation and differentiation of fibroblasts to myofibroblasts, increasing extracellular matrix (ECM) proteins and collagen deposition, ultimately leading to fibrosis (Fikry et al. 2015; Chanda et al. 2019). Pulmonary fibrosis is a heterogeneous lung condition characterized by excessive accumulation of scar tissue, leading to the destruction of lung architecture and restricted ventilation. Under physiological conditions, fibrogenesis is initiated due to tissue injury to resolve the wound area through four stages, including: coagulation, inflammation, fibroblast proliferation, and remodeling phases, where the normal structure of tissue and its integrity are renovated (Kant et al. 2021). The first FDA-approved medicine for the treatment of idiopathic pulmonary fibrosis (IPF) was pirfenidone (Pir), although the mechanism by which it acts is not entirely known (Hadjicharalambous and Lindsay 2020; Wilfong and Aggarwal 2021). Pir possesses antioxidant, anti-inflammatory, and antifibrotic properties as it reduced the level of TGF-β, IL-1β, IL-6, Interferon-γ (IFN-γ), MCP-1, and other cytokines in the bleomycin model of lung fibrosis (Hadjicharalambous and Lindsay 2020; Shah et al. 2021).
Cinnamon (Cinnamomum cassia) has a wide range of uses as a herbal medicine (Liu et al. 2020). The main chemical compound found in cinnamon is cinnamic acid (cin), which is one of the most common and basic phenolic acids found in nature (Hong et al. 2021; Ben Lagha et al. 2021). Cin possesses a lot of beneficial pharmacological actions such as antitumor, antimicrobial, antioxidant, and anti-inflammatory activities (El-Sayed et al. 2013; Ruwizhi and Aderibigbe 2020). Consequently, hindering inflammation and oxidative stress was deliberated as a prospective beneficial goal in the treatment of fibrotic diseases. The existent study aims to assess the potential shielding effect of cin, correlated to Pir, in contravention of pulmonary fibrotic-associated MTX use in rats.
Material
Animals
Male Sprague–Dawley rats (200–250 g) obtained from the animal house of the National Organization for Drug Control and Research (NODCAR, Cairo, Egypt) were used. Animals were lodged for at least one week in the laboratory room before testing under standard housing conditions: room temperature (24–27°C), humidity (60 ± 10%), alternating 12 h light and dark cycles, free access to food (standard pellet diet), and water ad libitum.
The investigation was permitted by the ethics committee for animal experimentation of the college of pharmacy, Cairo University, Egypt (PT 1511; approval date: October 26, 2015) and followed the instructions for the care and use of laboratory animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Drugs and chemicals
-
Dimethyl sulfoxide 50% (DMSO) was obtained from Sigma-Aldrich Corporation, Lyon, France, and was orally administrated in a dose of 5 ml/kg/day for 14 days.
-
MTX injection (50 mg/2 ml) was obtained from Mylan pharmaceutical Inc, USA, was orally administrated to induce lung fibrosis in a dose 14 mg/kg once weekly for 2 consecutive weeks.
-
Cin was provided from qualikems fine chemicals, New Delhi, Delhi, India, was orally given in a dose 50 mg/kg/day for 14 days. Cin was dissolved in 50% DMSO in concentration of (10 mg/ml).
-
Pir was obtained from Cipla LTD, Rorathang, Sikkim, India, was orally administered as a standard drug in a dose 50 mg/ kg/day for 14 days. Pir was dissolved in saline in concentration of (10 mg/ml).
Methods
Experimental design
Adult male Sprague–Dawley rats (200–250 g) were randomly dispersed into seven groups of 5–8 rats each and treated as follows:
-
Normal (saline) group: rats received saline orally daily.
-
Normal (DMSO) group: rats received 50% DMSO (solvent for cinnamic acid) orally daily (El-Sayed et al. 2013).
-
MTX group: rats received MTX, 14 mg/kg, orally once a week for 2 weeks; served as lung fibrotic group (Fikry et al. 2015).
-
Pir group: rats were treated with pir (50 mg/kg/day) orally; serve as a standard drug control group (Song et al. 2018).
-
Pir + MTX group: rats received pir (50 mg/kg/day) orally, whereas MTX (14 mg/kg) was orally given on the 5th and 12th days of the experiment; serve as a standard drug-treated group.
-
Cin group: rats were treated with cin (50 mg/kg/day) orally (El-Sayed et al. 2013).
-
Cin + MTX group: rats received cin in a dose of 50 mg/kg/day orally, whereas MTX (14 mg/kg) was orally given on the 5th and 12th days of the experiment.
The experiment lasted for 14 days; all solutions were given orally via a gavage needle.
At the finale, animals were decapitated, the lungs were erased, splashed with cold saline, blotted dry, and weighed. Three left lungs from different groups were conserved in 10% formalin for subsequent histopathological investigation. The used animals were frozen until they were incinerated.
-
N.B.: There is no significant alteration between the normal (saline) group and the normal (DMSO) group results in the existing study.
Preparation of lung homogenate
Lungs were homogenized in icy phosphate buffered saline, 1:10 w/v, and the homogenates were centrifuged at 13000 × g, 4°C, for 15 min. The supernatants were frozen at 80 °C in preparation for assessing the evaluated parameters.
Evaluated parameters
Evaluation of oxidative stress biomarkers in lung homogenate
The lung myeloperoxidase (MPO) was estimated using a myeloperoxidase colorimetric activity assay kit provided by Sigma-Aldrich Co. The lung nitric oxide (NO) was estimated using the reagents of the rat NO ELISA kit provided by Abbkine, Inc., China.
The malondialdehyde (MDA) and total glutathione (tGSH) contents in the lung were estimated using the reagents of lipid peroxidation and total glutathione assay kits, respectively, provided by Eagle Biosciences Inc., Boston. All the measures were carried out as specified in the constructors’ directives.
Assessment of inflammatory markers in lung homogenate
The lung TNF-α and TGF-β contents were estimated utilizing the commercially available rat ELISA kit provided by Mybiosource, San Diego, USA. The IL-8 content was assessed by consuming the commercially accessible rat IL-8 ELISA Kit provided by Cloud-Clone Corp., USA. These assays employ the quantitative sandwich enzyme immunoassay technique as reported by the manufacturers’ directives.
Western blot analysis of collagen in lung homogenate
Collagen content in the lung was evaluated by Clarity™ Western ECL substrate provided by Bio-Rad Laboratories, Inc., Canada.
The ReadyPrep™ protein extraction kit (total protein) provided by Bio-Rad Inc. was employed and added to lung samples. (Catalog #163–2086) and Bradford Protein Assay Kit (SK3041) for quantitative protein analysis was provided by Bio Basic Inc. (Markham, Ontario, L3R 8T4 Canada). A Bradford assay was performed according to the manufacturer's guidelines to evaluate every sample's protein content. After that, an equal volume of 2 × Laemmli sample buffer containing 4% SDS, 10% 2-mercaptoehtanol, 20% glycerol, 0.004% bromophenol blue, and 0.125 M Tris HCl was added to each sample's 20 g protein concentration. The pH was measured and adjusted to 6.8. Before loading on polyacrylamide gel electrophoresis, each prior mixture was heated at 95 °C for 5 min to achieve protein denaturation.
Protein separation by electrophoresis
Samples were separated on a polyacrylamide gel; the procedure was shortened to SDS-PAGE (Sodium Dodecyl Sulfate PolyAcrylamide Gel Electrophoresis), which is a standard technique for separating proteins according to their molecular weight. Polyacrylamide gels were performed using the TGX Stain-Free™ FastCast™ Acrylamide Kit (Bio-Rad Laboratories, Inc.).
Protein blotting (transfer of proteins from the gel to the membrane) and blocking the membrane
The gel was arranged in a transfer sandwich as follows from underneath to on top: filtration paper, PVDF membrane, gel, and filtration paper. It was positioned in the transfer tank with 1 × transfer buffer, which consists of 25 mM Tris, 190 mM glycine, and 20% methanol. The blot was run for 7 min at 25 V to allow protein bands to transfer from the gel to the membrane using BioRad Trans-Blot Turbo. The membrane was blocked in tris-buffered saline with Tween 20 (TBST) buffer and 3% bovine serum albumin (BSA) at 37 °C for 1 h. The blocking buffer consists of the following ingredients: 20 mM Tris pH 7.5, 150 mM NaCl, 0.1% Tween 20, and 3% bovine serum albumin (BSA).
Incubation with the primary antibody
Primary antibodies for collagen 1 A1 (Catalog Number: sc-293182, Santa Cruz Biotechnology, Inc., Europe) were diluted in TBST according to factory-made directions. Incubation was done overnight at 4 °C, against the blotted target protein. The blot was washed with TBST for 3–5 rounds for 5 min. In the HRP-conjugated secondary antibody solution (goat anti-rabbit IgG-HRP-1 mg goat mab, Novus Biologicals), the blotted target protein was incubated for 1 h at 37 °C. TBST was used to wash the blot 3–5 times for 5 min.
Imaging and data analysis quantitation
The chemiluminescent substrate (Clarity™ Western ECL substrate, Bio-Rad) was applied to the blot according to the manufacturer's instructions. Solution A (Clarity Western luminal/enhancer solution) and solution B (peroxidase solution) were mixed in equal parts. A CCD camera-based imager was used to capture the chemiluminescent signals. On the ChemiDoc MP imager, image analysis software was used to read the band intensity of the target proteins against the control sample, ꞵ-actin (housekeeping protein), by protein normalization.
Histopathological examination
Lung samples from various groups were maintained in 10% Formol saline, for 24 h. After washing the samples in tap water, they were dehydrated using serial dilutions of alcohol (methyl, ethyl, and absolute ethyl). In a hot air oven, specimens were cleared in xylene and embedded in paraffin for 24 h at 56 ºC. A sled microtome was used to segment paraffin-beeswax tissue blocks at 4 µ thickness. Tissue sections were collected on glass slides, deparaffinized, and stained with hematoxylin and eosin, in addition to Masson trichrome stain, for inspection under a light electric microscope.
Statistical analysis
Statistical analysis was achieved using instant automated software (Graph Pad Prism Software version 5.01, Inc., CA, USA). The results were implemented as the mean ± standard error of the mean (SEM). A one-way ANOVA followed by Tukey–Kramer multiple comparison tests was used. The results were deliberated significantly at P-value < 0.05.
Results
Evaluation of oxidative stress markers in lung homogenate
The present data displayed that MTX oral administration initiated a tremendous rise in oxidative stress, as shown by an upturn in MPO activity and NO lung content of 46% and 52%, respectively, as compared to the normal group. Similarly, an 8.6-fold intensification was detected in lung MDA levels after administration of MTX. Conversely, upon pre-treatment with cin, a 47% reduction in MPO activity was observed with a normalizing action in lung NO content and a noticeable drop in MDA content of 77%, related to the MTX control group. Using pir as a pre-treatment standard normalized MPO activity and significantly reduced the content of NO and MDA by 32% and 74%, respectively, relative to the MTX control group.
Meanwhile, a marked decrease in lung content of total GSH occurred in the MTX group by 66% in relation to normal rats. Upon pre-treatment with Cin or Pir, a significant elevation in the tGSH content by 5.4 and 3.4 folds, respectively, relative to the MTX control group was shown (Table 1).
Evaluation of inflammatory markers in lung homogenate
Oral treatment with MTX exhibited a significant increment in lung TNF-α content by 5.4-fold in comparison with normal rats. Oral pre-treatment administration of Cin instigated a significant reduction in TNF-α lung content of 0.4-fold. Meanwhile, TNF-α content reduced significantly in Pir pretreated rats by 0.3-fold in association with the MTX group (Fig. 1A).
Evaluation of inflammatory markers and Western blot analysis of Collagen in lung homogenate. (A): Assessment of TNF-α content, (B): Assessment of IL-8 content, (C): Assessment of TGF-β content, (D): Western blot analysis of Collagen in lung homogenate. Each value represents the mean of 5–8 rats ± SEM. Data were analyzed by One-way ANOVA followed by Tuckey-Kramer multiple comparison test. * P < 0.05 vs Normal group, # P < 0.05 vs MTX group, p P < 0.05 vs Pir + MTX
Rats receiving MTX displayed a significant augmentation in IL-8 content by 3.8-fold compared to normal rats. Rats pretreated with cin and pir reduced lung content of IL-8 by approximately 62% compared to the MTX group (Fig. 1B).
Lung content of TGF-β was significantly increased following MTX administration by 3.8-fold as compared to the normal group. Conversely, a marked reduction in TGF-β content by 61% was presented in the cin-pretreated group. Likewise, the results revealed that oral administration of pir before MTX reduced the lung content of TGF-β by 67% relative to the MTX group (Fig. 1C).
Western blot analysis of collagen in lung homogenate
A significant boost of the lung collagen content by 4.3-fold was obtained due to oral administration of MTX, linked to normal rats. However, a 67% and 61% depletion of the collagen content were detected in cin- and pir-pretreated rats, respectively, linked to the MTX control group (Fig. 1D).
Histopathological examination
Hematoxylin and eosin (H&E) stain
In the lung of the normal (saline) group, there was no histopathological alteration recorded (Fig. 2A).
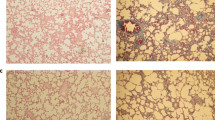
Photomicrographs of rat lung sections stained with H&E (X 16). (A) Normal group: normal histological structure of the bronchioles and surrounding air alveoli; (B) MTX treated group: there was fibrosis and collagen proliferation in the peribronchiolar area; (C) Pir treated group: few inflammation cells infiltration in perivascular tissue; (D) Pir + MTX treated group: few inflammation cells infiltration in peribronchiolar tissue; (E) Cin treated group: no histopathological alteration; (F) Cin + MTX treated group: sclerosis of the vascular wall
Rats subjected to MTX showed peribronchiolar tissue and lymphoid follicle hyperplasia associated with dilatation in the blood vessels and inflammatory cell infiltration in the parenchyma (Fig. 2B). Treatment with cin alone showed a normal appearance of cells (Fig. 2E). However, the cin + MTX group exhibited thickening and hypertrophy of the vascular wall (Fig. 2F).
There was no histopathological alteration in the parenchyma, but the perivascular tissue presented few inflammatory cell infiltrations in the perivascular tissue of the pir control group (Fig. 2C). Moreover, the peribronchiolar tissue in rats subjected to MTX after pir treatment exhibited a thickened wall and few inflammatory cell infiltrations (Fig. 2D).
Masson’s trichrome stain
Severe proliferation of collagen and fibroblastic cells was revealed in the lung of MTX-received rats (Fig. 3B). Conversely, pre-treatment with either cin or pir in MTX-treated rats showed improvement with less appearance of fibrosis in the peribronchiolar and perivascular areas upon using Masson’s trichrome stain (Fig. 3D–F).
Discussion
MTX is an antimetabolite drug used to treat types of cancer and rheumatoid arthritis through its antiproliferative properties (Juge et al. 2021). Several suggested pharmacological mechanisms of MTX action are suppression of thymidine and purine biosynthesis, inhibition of lymphoid tissue proliferation, particularly T lymphocytes, and initiation of the MAPK pathway, resulting in the motivation of activator protein-1 and nuclear factor-κB, which are fateful for inflammation regulation (Yan et al. 2021). Moreover, through the activation of oxidation systems, performed as an unneeded amendment in oxidative stress markers (MPO, MDA, and GSH), inflammatory cells penetrate the tissue, causing injury and cell death by generating DNA damage (Türk et al. 2022; Elsawy et al. 2021).
The present results revealed that oral administration of MTX produced lung oxidative stress alteration, as shown by a significant promotion in MPO activity as well as NO and MDA contents, versus a noticeable decline in lung content of total GSH. These outcomes are reliable in Al-Taher et al. (2020); Roghani et al. (2020); Sherif et al. (2020); and Jafaripour et al. (2021).
It was evident that MTX caused pulmonary toxicity due to its direct action via increased ROS production, leading to the activation of MPO, a lysosomal enzyme found in neutrophils responsible for hypochlorous acid production from hydrogen peroxide and chloride ions. The ROS production upturn, due to MTX, leads to NO and MDA intensification. NO induces the versatile oxidant peroxynitrite through a reaction with superoxide anions (Abouelela et al. 2019).
Moreover, the increase in MDA, the end product of polyunsaturated fatty acids, indicates lipid peroxidation (Roghani et al. 2020). While the oxidizing factors (MPO, MDA, and NO) were raised in lung tissue, the antioxidant factors (tGSH) were diminished during MTX administration, leading to an imbalance between the production of ROS and its elimination by antioxidant defenses (Mammadov et al. 2019; Ozcicek et al. 2020 and Mansour et al. 2021).
Additionally, the MTX-induced lung inflammation and fibrosis were revealed by a manifest escalation in the lung TNF-α, IL-8, TGF-β, and collagen contents. The significant elevation of inflammatory markers in addition to collagen as a fibrotic marker during MTX treatment was in harmony with Yamagami et al. (2020); Mohamed et al. (2021); Taskin et al. (2021) and Zaki et al. (2021). These actions could be attributed to the MTX’s ability to activate the leukocytes to increase the liberation of cytokines, which have a fundamental role in lung fibrosis formation through the epithelial-mesenchymal transition process and stimulation of NF-kβ and activator platelet-1 through ROS generation (Al Kury et al. 2020).
Conversely, the existing study demonstrated that the cin pre-treatment raised tGSH lung content, combined with a significant reduction in MPO activity as well as NO, MDA contents, TNF-α, IL-8, TGF-β, and collagen.
These observations are due to the antioxidant and anti-inflammatory properties of cin (Karatas et al. 2020; Babaeenezhad et al. 2021). It has been shown that cin derivatives suppress MAPK and AKT signaling pathways, causing NF-kβ, activator protein-1 attenuation (Abozaid et al. 2020; Godlewska-Żyłkiewicz et al. 2020; Hazafa et al. 2022). In agreement with our data, Abozaid et al. (2020) demonstrated the modulating effects of cinnamic acid on the redox signal and inflammatory response in an acute pancreatitis model, and Yan et al. (2018) referred to cinnamaldehyde's influence on IPF in mice via protesting against inflammation and oxidative stress. Besides, Ibrahim et al. (2020) discovered that cinnamic acid nanoparticles suppress apoptosis in an acute hepatitis rat model.
Pir was approved as the first therapy for mild and moderate IPF patients in the EU and USA as it has antioxidant, anti-inflammatory, and antifibrotic accomplishments (Tzouvelekis et al. 2017; Kreuter et al. 2021). Pir was taken in this study as a reference drug. Our results demonstrated that oral pre-treatment with pir enhanced the activity of tGSH. While reducing the activity of MPO, NO, and MDA contents, it also diminished TNF-α, IL-8, TGF-β, and collagen contents in the lung. The upshots match Ballester et al. (2020); Long et al. (2020) and Seifirad (2020).
In parallel, the histopathological examination showed that MTX caused a severe proliferation of collagen and fibroblastic cells, which indicates its fibrotic effect. Contrariwise, the effect of cin or pir pre-treatment mitigated the lung fibrosis caused by MTX administration.
Following pre-treatment with cin, our study revealed a notable reduction in MPO, NO, and MDA levels, along with an increase in tGSH level. This suggests that cin may hinder the activation of the JNK-signaling pathway while suppressing the effects of epidermal growth factor (EGF) and PDGF. Additionally, the decrease in TNF-alpha and IL-8, in conjunction with TGF-beta, may alleviate the activation of ERK and p38 mitogen-activated protein kinase signaling pathways. This would result in negative regulation of platelet aggregation and a decrease in the activation and proliferation of fibroblasts and collagen, indicating the advantageous impact of cin on MTX-induced lung fibrosis.
In conclusion, the present study revealed that pre-treatment with cinnamic acid caused a discernible escalation in tGSH content, combined with a significant diminution in MPO activity as well as NO and MDA contents, in addition to TNF-α, IL-8, TGF-β, and collagen. Optimistically, the data obtained from the present study showed a great resemblance between cin and pir, as the outcomes of cin administration were more or less the same as those of pir. Therefore, cin could be used as an adjuvant therapy with MTX, as it is a promising prophylactic treatment for lung fibrosis induced by MTX. The prophylactic effect of cinnamic acid against lung fibrosis is allied to its antioxidant, anti-inflammatory, and anti-fibrotic properties.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abouelela ME, Orabi MAA, Abdelhamid RA, Abdelkader MS, Madkor HR, Darwish FMM, Hatano T, Elsadek BEM (2019) Ethyl acetate extract of Ceiba pentandra (L.) Gaertn. reduces methotrexate-induced renal damage in rats via antioxidant, anti-inflammatory, and antiapoptotic actions. J Tradit Complement Med 10(5):478–486
Abozaid OAR, Moawed FSM, Ahmed ESA, Ibrahim ZA (2020) Cinnamic acid nanoparticles modulate redox signal and inflammatory response in gamma irradiated rats suffering from acute pancreatitis. Biochim Biophys Acta Mol Basis Dis 1866(11):165904
Al Kury LT, Dayyan F, Ali Shah F, Malik Z, Khalil AAK, Alattar A, Alshaman R, Ali A, Khan Z (2020) Ginkgo biloba Extract Protects against Methotrexate-Induced Hepatotoxicity: A Computational and Pharmacological Approach. Molecules 25(11):2540
Al-Taher AY, Morsy MA, Rifaai RA, Zenhom NM, Abdel-Gaber SA (2020) Paeonol Attenuates Methotrexate-Induced Cardiac Toxicity in Rats by Inhibiting Oxidative Stress and Suppressing TLR4-Induced NF-κB Inflammatory Pathway. Mediators Inflamm 2020:8641026
Babaeenezhad E, Nouryazdan N, Nasri M, Ahmadvand H, Moradi Sarabi M (2021) Cinnamic acid ameliorate gentamicin-induced liver dysfunctions and nephrotoxicity in rats through induction of antioxidant activities. Heliyon 7(7):e07465
Ballester B, Milara J, Cortijo J (2020) Pirfenidone anti-fibrotic effects are partially mediated by the inhibition of MUC1 bioactivation. Oncotarget 11(15):1306–1320
Ben Lagha A, Azelmat J, Vaillancourt K, Grenier D (2021) A polyphenolic cinnamon fraction exhibits anti-inflammatory properties in a monocyte/macrophage model. PLoS ONE 16(1):e0244805
Chanda D, Otoupalova E, Smith SR, Volckaert T, De Langhe SP, Thannickal VJ (2019) Developmental pathways in the pathogenesis of lung fibrosis. Mol Aspects Med 65:56–69
Chen J, Wang J, Li C, Ding H, Ye J, Xia Z (2021) Dexmedetomidine reverses MTX-induced neurotoxicity and inflammation in hippocampal HT22 cell lines via NCOA4-mediated ferritinophagy. Aging (albany NY) 13(4):6182–6193
Dong WC, Guo JL, Wu XK, Zhao MQ, Li HR, Zhang ZQ, Jiang Y (2021) Relationship Between the Free and Total Methotrexate Plasma Concentration in Children and Application to Predict the Toxicity of HD-MTX. Front Pharmacol 12:636975
Elsawy H, Alzahrani AM, Alfwuaires M, Abdel-Moneim AM, Khalil M (2021) Nephroprotective effect of naringin in methotrexate induced renal toxicity in male rats. Biomed Pharmacother 143:112180
El-Sayed M, Abd El-Raouf OM, Fawzy HM, Manie MF (2013) Comparative study of the possible prMotective effects of cinnamic acid and cinnamaldehyde on cisplatin-induced nephrotoxicity in rats. J Biochem Mol Toxicol 27(12):508–14
Fikry EM, Safar MM, Hasan WA, Fawzy HM, El-Denshary EE (2015) Bone Marrow and Adipose-Derived Mesenchymal Stem Cells Alleviate Methotrexate-Induced Pulmonary Fibrosis in Rat: Comparison with Dexamethasone. J Biochem Mol Toxicol 29(7):321–329
Ghoneum M, El-Gerbed MSA (2021) Human placental extract ameliorates methotrexate-induced hepatotoxicity in rats via regulating antioxidative and anti-inflammatory responses. Cancer Chemother Pharmacol 88(6):961–971
Godlewska-Żyłkiewicz B, Świsłocka R, Kalinowska M, Golonko A, Świderski G, Arciszewska Ż, Nalewajko-Sieliwoniuk E, Naumowicz M, Lewandowski W (2020) Biologically Active Compounds of Plants: Structure-Related Antioxidant, Microbiological and Cytotoxic Activity of Selected Carboxylic Acids. Materials (basel) 13(19):4454
Hadjicharalambous MR, Lindsay MA (2020) Idiopathic Pulmonary Fibrosis: Pathogenesis and the Emerging Role of Long Non-Coding RNAs. Int J Mol Sci 21(2):524
Hazafa A, Iqbal MO, Javaid U, Tareen MBK, Amna D, Ramzan A, Piracha S, Naeem M (2022) Inhibitory effect of polyphenols (phenolic acids, lignans, and stilbenes) on cancer by regulating signal transduction pathways: a review. Clin Transl Oncol 24(3):432–445
Hong S, Cha KH, Park JH, Jung DS, Choi JH, Yoo G, Nho CW (2021) Cinnamic acid suppresses bone loss via induction of osteoblast differentiation with alteration of gut microbiota. J Nutr Biochem 101:108900
Howard SC, McCormick J, Pui CH, Buddington RK, Harvey RD (2016) Preventing and Managing Toxicities of High-Dose Methotrexate. Oncologist 21(12):1471–1482
Ibrahim EA, Moawed FSM, Moustafa EM (2020) Suppression of inflammatory cascades via novel cinnamic acid nanoparticles in acute hepatitis rat model. Arch Biochem Biophys 696:108658
Jafaripour L, Naserzadeh R, Alizamani E, Javad Mashhadi SM, Moghadam ER, Nouryazdan N, Ahmadvand H (2021) Effects of Rosmarinic Acid on Methotrexate-induced Nephrotoxicity and Hepatotoxicity in Wistar Rats. Indian J Nephrol 31(3):218–224
Jani M, Dixon WG, Matteson EL (2017) Management of the Rheumatoid Arthritis Patient with Interstitial Lung Disease. Lung Dis Rheumatoid Arthritis 121–161
Juge PA, Lee JS, Lau J, Kawano-Dourado L, Rojas Serrano J, Sebastiani M, Koduri G, Matteson E, Bonfiglioli K, Sawamura M, Kairalla R, Cavagna L, Bozzalla Cassione E, Manfredi A, Mejia M, Rodríguez-Henriquez P, González-Pérez MI, Falfán-Valencia R, Buendia-Roldán I, Pérez-Rubio G, Ebstein E, Gazal S, Borie R, Ottaviani S, Kannengiesser C, Wallaert B, Uzunhan Y, Nunes H, Valeyre D, Saidenberg-Kermanac’h N, Boissier MC, Wemeau-Stervinou L, Flipo RM, Marchand-Adam S, Richette P, Allanore Y, Dromer C, Truchetet ME, Richez C, Schaeverbeke T, Lioté H, Thabut G, Deane KD, Solomon JJ, Doyle T, Ryu JH, Rosas I, Holers VM, Boileau C, Debray MP, Porcher R, Schwartz DA, Vassallo R, Crestani B, Dieudé P (2021) Methotrexate and rheumatoid arthritis associated interstitial lung disease. Eur Respir J 57(2):2000337
Kalemci S, Akpınar O, Dere Y, Sarıhan A, Zeybek A, Tanriverdi Ö (2018) Efficacy of clarithromycin as a protective agent in the methotrexate-induced pulmonary fibrosis model. Kardiochir Torakochirurgia Pol 15(4):209–212
Kant TA, Newe M, Winter L, Hoffmann M, Kämmerer S, Klapproth E, Künzel K, Kühnel MP, Neubert L, El-Armouche A, Künzel SR (2021) Genetic Deletion of Polo-Like Kinase 2 Induces a Pro-Fibrotic Pulmonary Phenotype. Cells 10(3):617
Karatas O, Balci Yuce H, Taskan MM, Gevrek F, Alkan C, Isiker Kara G, Temiz C (2020) Cinnamic acid decreases periodontal inflammation and alveolar bone loss in experimental periodontitis. J Periodontal Res 55(5):676–685
Koppelmann T, Pollak Y, Ben-Shahar Y, Gorelik G, Sukhotnik I (2021) The Mechanisms of the Anti-Inflammatory and Anti-Apoptotic Effects of Omega-3 Polyunsaturated Fatty Acids during Methotrexate-Induced Intestinal Damage in Cell Line and in a Rat Model. Nutrients 13(3):888
Kreuter M, Lee JS, Tzouvelekis A, Oldham JM, Molyneaux PL, Weycker D, Atwood M, Kirchgaessler KU, Maher TM (2021) Monocyte Count as a Prognostic Biomarker in Patients with Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 204(1):74–81
Liu P, Wang J, Wen W, Pan T, Chen H, Fu Y, Wang F, Huang JH, Xu S (2020) Cinnamaldehyde suppresses NLRP3 derived IL-1β via activating succinate/HIF-1 in rheumatoid arthritis rats. Int Immunopharmacol 84:106570
Long X, Tang Y, Li J, Wang W, Ou D, Xu L, He J, Xie L (2020) Pirfenidone Inhibits Cytokines/chemokines Release from Alveolar Macrophages. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 42(5):596–602 (Chinese)
Mammadov R, Suleyman B, Akturan S, Cimen FK, Kurt N, Suleyman Z, Malkoc İ (2019) Effect of lutein on methotrexate-induced oxidative lung damage in rats: a biochemical and histopathological assessment. Korean J Intern Med 34(6):1279–1286
Mansour DF, Saleh DO, Ahmed-Farid OA, Rady M, Bakeer RM, Hashad IM (2021) Ginkgo biloba extract (EGb 761) mitigates methotrexate-induced testicular insult in rats: Targeting oxidative stress, energy deficit and spermatogenesis. Biomed Pharmacother 143:112201
Mohamed M, El Sheikh AK, Mohammed HH (2021) Modulation of Liver P-Glycoprotien Expression May Contribute to Gossypin Protection against Methotrexate-Induced Hepatotoxicity. Indian J Pharmacol 53(1):25–30
Olsen NJ, Spurlock CF 3rd, Aune TM (2014) Methotrexate induces production of IL-1 and IL-6 in the monocytic cell line U937. Arthritis Res Ther 16(1):R17
Ozcicek F, Kara AV, Akbas EM, Kurt N, Yazici GN, Cankaya M, Mammadov R, Ozcicek A, Suleyman H (2020) Effects of anakinra on the small intestine mucositis induced by methotrexate in rats. Exp Anim 69(2):144–152
Rana RM, Rampogu S, Abid NB, Zeb A, Parate S, Lee G, Yoon S, Kim Y, Kim D, Lee KW (2020) In Silico Study Identified Methotrexate Analog as Potential Inhibitor of Drug Resistant Human Dihydrofolate Reductase for Cancer Therapeutics. Molecules 25(15):3510
Roghani M, Kalantari H, Khodayar MJ, Khorsandi L, Kalantar M, Goudarzi M, Kalantar H (2020) Alleviation of Liver Dysfunction, Oxidative Stress and Inflammation Underlies the Protective Effect of Ferulic Acid in Methotrexate-Induced Hepatotoxicity. Drug Des Devel Ther 14:1933–1941
Ruwizhi N, Aderibigbe BA (2020) Cinnamic Acid Derivatives and Their Biological Efficacy. Int J Mol Sci 21(16):5712
Seifirad S (2020) Pirfenidone: A novel hypothetical treatment for COVID-19. Med Hypotheses 144:110005
Shah PV, Balani P, Lopez AR, Nobleza CMN, Siddiqui M, Khan S (2021) A Review of Pirfenidone as an Anti-Fibrotic in Idiopathic Pulmonary Fibrosis and Its Probable Role in Other Diseases. Cureus 13(1):e12482
Sherif IO, Al-Mutabagani LA, Sarhan OM (2020) Ginkgo biloba Extract Attenuates Methotrexate-Induced Testicular Injury in Rats: Cross-talk Between Oxidative Stress, Inflammation, Apoptosis, and miRNA-29a Expression. Integr Cancer Ther 19:1534735420969814
Song X, Yu W, Guo F (2018) Pirfenidone suppresses bleomycin-induced pulmonary fibrosis and periostin expression in rats. Exp Ther Med 16(3):1800–1806
Taskin B, Erdoğan MA, Yiğittürk G, Alper S, Erbaş O (2021) An Experimental Study: Benefits of Digoxin on Hepatotoxicity Induced by Methotrexate Treatment. Gastroenterol Res Pract 2021:6619844
Türk E, Güvenç M, Cellat M, Uyar A, Kuzu M, Ağgül AG, Kırbaş A (2022) Zingerone protects liver and kidney tissues by preventing oxidative stress, inflammation, and apoptosis in methotrexate-treated rats. Drug Chem Toxicol 45(3):1054–1065
Tzouvelekis A, Karampitsakos T, Ntolios P, Tzilas V, Bouros E, Markozannes E, Malliou I, Anagnostopoulos A, Granitsas A, Steiropoulos P, Dimakou K, Chrysikos S, Koulouris N, Longitudinal BD (2017) “Real-World” Outcomes of Pirfenidone in Idiopathic Pulmonary Fibrosis in Greece. Front Med (lausanne) 4:213
Wilfong EM, Aggarwal R (2021) Role of antifibrotics in the management of idiopathic inflammatory myopathy associated interstitial lung disease. Ther Adv Musculoskelet Dis 13:1759720X211060907
Yamagami Y, Kawami M, Ojima T, Futatsugi S, Yumoto R, Takano M (2020) Role of plasminogen activator inhibitor-1 in methotrexate-induced epithelial-mesenchymal transition in alveolar epithelial A549 cells. Biochem Biophys Res Commun 525(3):543–548
Yan L, Song F, Li H, Li Y, Li J, He QY, Zhang D, Wang F, Zhang M, Zhao H, Feng T, Zhao YY, Wang SW (2018) Submicron emulsion of cinnamaldehyde ameliorates bleomycin-induced idiopathic pulmonary fibrosis via inhibition of inflammation, oxidative stress and epithelial-mesenchymal transition. Biomed Pharmacother 102:765–771
Yan H, Su R, Xue H, Gao C, Li X, Wang C (2021) Pharmacomicrobiology of Methotrexate in Rheumatoid Arthritis: Gut Microbiome as Predictor of Therapeutic Response. Front Immunol 12:789334
Zaki SM, Hussein GHA, Khalil HMA, Abd Algaleel WA (2021) Febuxostat ameliorates methotrexate-induced lung damage. Folia Morphol (warsz) 80(2):392–402
Acknowledgements
The authors thank Professor A. Bakear (Pathology Department, Faculty of Veterinary Medicine, Cairo, Egypt) for his aid in the histopathological inspections.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
E.A., R. A., S. K. designed the study and the conceptualization. E. A., G.G. performed the study, Investigation and formal analysis. E. A., E. A., G. G., H. F., S. K Wrote, reviewed and edited the manuscript. The authors declare that all data were generated in‑house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
The investigation was permitted by the ethics committee for animal experimentation of the college of pharmacy, Cairo University, Egypt (PT 1511), (Approval Date: 26/10/2015) and followed the instructions for the care and use of laboratory animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdalhameid, E., Abd El-Haleim, E.A., Abdelsalam, R.M. et al. Cinnamic acid mitigates methotrexate-induced lung fibrosis in rats: comparative study with pirfenidone. Naunyn-Schmiedeberg's Arch Pharmacol 397, 1071–1079 (2024). https://doi.org/10.1007/s00210-023-02652-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02652-w