Abstract
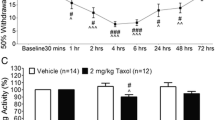
In this study, we determined the therapeutic effect of parthenolide (PTL), the active component of Tanacetum parthenium, on neuropathic pain caused by paclitaxel (PTX), a chemotherapeutic drug frequently used in cancer treatment, at the gene and protein levels. To this end, 6 groups were formed: control, PTX, sham, 1 mg/PTL, 2 mg/kg PTL, and 4 mg/kg PTL. Pain formation was tested by Randall-Selitto analgesiometry and locomotor activity behavioral analysis. Then, PTL treatment was performed for 14 days. After the last dose of PTL was taken, Hcn2, Trpa1, Scn9a, and Kcns1 gene expressions were measured in rat brain (cerebral cortex/CTX) tissues. In addition, changes in the levels of SCN9A and KCNS1 proteins were determined by immunohistochemical analysis. Histopathological hematoxylin-eosin staining was also performed to investigate the effect of PTL in treating tissue damage on neuropathic pain caused by PTX treatment. When the obtained data were analyzed, pain threshold and locomotor activity decreased in PTX and sham groups and increased with PTL treatment. In addition, it was observed that the expression of the Hcn2, Trpa1, and Scn9a genes decreased while the Kcns1 gene expression increased. When protein levels were examined, it was determined that SCN9A protein expression decreased and the KCNS1 protein level increased. It was determined that PTL treatment also improved PTX-induced tissue damage. The results of this study demonstrate that non-opioid PTL is an effective therapeutic agent in the treatment of chemotherapy-induced neuropathic pain, especially when used at a dose of 4 mg/kg acting on sodium and potassium channels.









Similar content being viewed by others
Data availability
The data supporting the findings of the present research are available on request from the corresponding author upon reasonable request.
References
Addington J, Freimer M (2016) Chemotherapy-induced peripheral neuropathy: an update on the current understanding. F1000Res 5. https://doi.org/10.12688/f1000research.8053.1
Aksu EH, Özkaraca MUSTAFA, Kandemir FM, Ömür AD, Eldutar E, Küçükler S, Çomaklı S (2016) Mitigation of paracetamol‐induced reproductive damage by chrysin in male rats via reducing oxidative stress. Andrologia 48(10):1145–1154
Alessandri-Haber N, Dina OA, Joseph EK, Reichling DB, Levine JD (2008) Interaction of transient receptor potential vanilloid 4, integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J Neurosci 28(5):1046–1057. https://doi.org/10.1523/JNEUROSCI.4497-07.2008
Alves CJ, Couto M, Sousa DM, Magalhães A, Neto E, Leitão L, Conceição F, Monteiro AC, Ribeiro-da-Silva M, Lamghari M (2020) Nociceptive mechanisms driving pain in a post-traumatic osteoarthritis mouse model. Sci Rep 10(1):15271. https://doi.org/10.1038/s41598-020-72227-9
Anderson LM, Samineni S, Wilder DM, Lara M, Eken O, Urioste R, Long JB, Arun P (2021) The neurobehavioral effects of buprenorphine and meloxicam on a blast-ınduced traumatic brain ınjury model in the rat. Front Neurol 12:74637012. https://doi.org/10.3389/fneur.2021.746370
Barabási A-L, Oltvai ZN (2004) Network biology: understanding the cell’s functional organization. Nat Rev Genet 5(2):101–113. https://doi.org/10.1038/nrg1272
Benzer F, Kandemir FM, Ozkaraca M, Kucukler S, Caglayan C (2018) Curcumin ameliorates doxorubicin-induced cardiotoxicity by abrogation of inflammation, apoptosis, oxidative DNA damage, and protein oxidation in rats. J Biochem Mol Toxicol 32(2):e22030
Bernard Healey SA, Scholtes I, Abrahams M, McNaughton PA, Menon DK, Lee MC (2021) Role of hyperpolarization-activated cyclic nucleotide-gated ion channels in neuropathic pain: a proof-of-concept study of ivabradine in patients with chronic peripheral neuropathic pain. Paın Rep 6(4):e967. https://doi.org/10.1097/PR9.0000000000000967
Bouhassira D, Attal N (2018) Emerging therapies for neuropathic pain: new molecules or new indications for old treatments? Pain 159(3):576–582. https://doi.org/10.1097/j.pain.0000000000001136
Bree D, Moriarty O, Broom DC, Kelly JP, Roche M, Finn DP (2016) Characterization of the affective component of acute postoperative pain associated with a novel rat model of ınguinal hernia repair pain. CNS Neurosci Ther 22(2):146–153. https://doi.org/10.1111/cns.12483
Budak H, Ceylan H, Kocpinar EF, Gonul N, Erdogan O (2014) Expression of Glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in oxidative stress ınduced by long- term ıron toxicity in rat liver. J Biochem Mol Toxicol 28(5):217–223. https://doi.org/10.1002/jbt.21556
Busserolles J, Tsantoulas C, Eschalier A, García JAL (2016) Potassium channels in neuropathic pain: advances, challenges, and emerging ideas. Pain 157:S7–S14
Cai W, Zhao Q, Shao J, Zhang J, Li L, Ren X, Zang W (2018) MicroRNA-182 alleviates neuropathic pain by regulating Nav1. 7 following spared nerve injury in rats. Sci Rep 8(1):1–11
Canta A, Pozzi E, Carozzi VA (2015) Mitochondrial dysfunction in chemotherapy-ınduced peripheral neuropathy (CIPN). Toxics 3(2):198–223. https://doi.org/10.3390/toxics3020198
Ceylan H, Budak H, Kocpinar EF, Baltaci NG, Erdogan O (2019) Examining the link between dose-dependent dietary iron intake and Alzheimer’s disease through oxidative stress in the rat cortex. J Trace Elem Med Biol 56:198–206. https://doi.org/10.1016/j.jtemb.2019.09.002
Chung JM, Chung K (2004) Sodium channels and neuropathic pain. Pathological Pain: From Molecular to Clinical Aspects 261:19–27
Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Raja SN (2017) Neuropathic pain. Nat Rev Dis Primers 3(1):1–19
Colvin LA (2019) Chemotherapy-induced peripheral neuropathy (CIPN): where are we now? Pain 160(Suppl 1):S1–S10. https://doi.org/10.1097/j.pain.0000000000001540
Costigan (2009) Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 32:1–32
Costigan M, Belfer I, Griffin RS, Dai F, Barrett LB, Coppola G, Wu TX, Kiselycznyk C, Poddar M, Lu Y, Diatchenko L, Smith S, Cobos EJ, Zaykin D, Allchorne A, Shen PH, Nikolajsen L, Karppinen J, Mannikko M, … Woolf CJ (2010) Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain 133:2519–2527. https://doi.org/10.1093/brain/awq195
Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, Al-Gazali L, Hamamy H, Valente EM, Gorman S, Williams R, McHale DP, Wood JN, Gribble FM, Woods CG (2006) An SCN9A channelopathy causes congenital inability to experience pain. Nature 444(7121):894–898. https://doi.org/10.1038/nature05413
Dabby R, Sadeh M, Gilad R, Lampl Y, Cohen S, Inbar S, Leshinsky-Silver E (2011) Chronic non-paroxysmal neuropathic pain—novel phenotype of mutation in the sodium channel SCN9A gene. J Neurol Sci 301(1–2):90–92
Deuis JR, Dvorakova LS, Vetter I (2017) Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci 10:28410. https://doi.org/10.3389/fnmol.2017.00284
Dib-Hajj SD, Cummins TR, Black JA, Waxman SG (2007) From genes to pain: Na(v)1.7 and human pain disorders. Trends Neurosci 30(11):555–563. https://doi.org/10.1016/j.tins.2007.08.004
Dini L, Del Lungo M, Resta F, Melchiorre M, Spinelli V, Di Cesare Mannelli L, Ghelardini C, Laurino A, Sartiani L, Coppini R, Mannaioni G, Cerbai E, Romanelli MN (2018) Selective blockade of HCN1/HCN2 channels as a potential pharmacological strategy against pain. Front Pharmacol 9:1252. https://doi.org/10.3389/fphar.2018.01252
Farquhar-Smith P (2011) Chemotherapy-induced neuropathic pain. Curr Opin Support Palliat Care 5(1):1–7
Fernandes ES, Russell FA, Spina D, McDougall JJ, Graepel R, Gentry C, Staniland AA, Mountford DM, Keeble JE, Malcangio M, Bevan S, Brain SD (2011) A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor α-induced inflammatory hyperalgesia and Freund’s complete adjuvant-induced monarthritis. Arthritis Rheum 63(3):819–829. https://doi.org/10.1002/art.30150
Fiebich (2002) Inhibition of LPS-induced p42/44 MAP kinase activation and iNOS/NO synthesis by parthenolide in rat primary microglial cells. J Neuroimmunol 132:(1-2):18–24
Fields HL (2011) The doctor’s dilemma: opiate analgesics and chronic pain. Neuron 69(4):591–594. https://doi.org/10.1016/j.neuron.2011.02.001
Furgała A, Fijałkowski Ł, Nowaczyk A, Sałat R, Sałat K (2018) Time-shifted co-administration of sub-analgesic doses of ambroxol and pregabalin attenuates oxaliplatin-induced cold allodynia in mice. Biomed Pharmacother 106:930–940
Gao W, Zan Y, Wang ZJJ, Hu XY, Huang F (2016) Quercetin ameliorates paclitaxel-induced neuropathic pain by stabilizing mast cells, and subsequently blocking PKCε-dependent activation of TRPV1. Acta Pharmacol Sin 37(9):1166–1177
Gerdle B, Ghafouri B (2020) Proteomic studies of common chronic pain conditions - a systematic review and associated network analyses. Expert Rev Proteomics 17(6):483–505. https://doi.org/10.1080/14789450.2020.1797499
Goldlust SA, Kavoosi M, Nezzer J, Kavoosi M, Korz W, Deck K (2021) Tetrodotoxin for chemotherapy-ınduced neuropathic pain: a randomized, double-blind, placebo-controlled, parallel-dose finding trial. Toxins 13(4). ARTN 235. https://doi.org/10.3390/toxins13040235
Gomez-Varela D, Barry AM, Schmidt M (2019) Proteome-based systems biology in chronic pain. J Proteomics 190:1–11. https://doi.org/10.1016/j.jprot.2018.04.004
Hargus NJ, Patel MK (2007) Voltage-gated Na+ channels in neuropathic pain. Expert Opin Investig Drugs 16(5):635–646
Hasriadi, Wasana PWD, Vajragupta O, Rojsitthisak P, Towiwat P (2021) Automated home-cage for the evaluation of innate non-reflexive pain behaviors in a mouse model of inflammatory pain. Sci Rep 11(1):12240. https://doi.org/10.1038/s41598-021-91444-4
Hendry L, Lombard Z, Wadley A, Kamerman P (2013) KCNS1, but not GCH1, ıs associated with pain ıntensity in a black southern african population with HIV-Associated sensory neuropathy: a genetic association study. Jaids-J Acquir Immune Defic Syndr 63(1):27–30. https://doi.org/10.1097/QAI.0b013e318285cf36
Huynh PN, Giuvelis D, Christensen S, Tucker KL, McIntosh JM (2019) RgIA4 accelerates recovery from paclitaxel-ınduced neuropathic pain in rats. Mar Drugs 18(1):12. https://doi.org/10.3390/md18010012
Huynh PN, Giuvelis D, Christensen S, Tucker KL, McIntosh JM (2020) RgIA4 accelerates recovery from paclitaxel-ınduced neuropathic pain in rats. Mar Drugs 18(1). ARTN 12. https://doi.org/10.3390/md18010012
Ibrahim SA, Albany Z, Albany C (2015) Significant response to lacosamide in a patient with severe chemotherapy-induced peripheral neuropathy. J Community Support Oncol 13(5):202–204
Jain NK, Kulkarni SK (1999) Antinociceptive and anti-inflammatory effects of Tanacetum parthenium L. extract in mice and rats. J Ethnopharmacol 68(1–3):251–259. https://doi.org/10.1016/s0378-8741(99)00115-4
Jansen L-AR, Forster LA, Smith XL, Rubaharan M, Murphy AZ, Baro DJ (2021) Changes in peripheral HCN2 channels during persistent inflammation. Channels 15(1):164–178. https://doi.org/10.1080/19336950.2020.1870086
Katsura H, Obata K, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Sakagami M, Noguchi K (2006) Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp Neurol 200(1):112–123. https://doi.org/10.1016/j.expneurol.2006.01.031
Kocpinar EF, Baltaci NG, Ceylan H, Kalin SN, Erdogan O, Budak H (2020) Effect of a prolonged dietary ıron ıntake on the gene expression and activity of the testicular antioxidant defense system in rats. Biol Trace Elem Res 195(1):135–141. https://doi.org/10.1007/s12011-019-01817-0
Kuzmanov U, Emili A (2013) Protein-protein interaction networks: probing disease mechanisms using model systems. Genome Med 5(4):37. https://doi.org/10.1186/gm441
Langford DJ, West C, Elboim C, Cooper BA, Abrams G, Paul SM, Schmidt BL, Levine JD, Merriman JD, Dhruva A, Neuhaus J, Leutwyler H, Baggott C, Sullivan CW, Aouizerat BE, Miaskowski C (2014) Variations in potassium channel genes are associated with breast pain in women prior to breast cancer surgery. J Neurogenet 28(1–2):122–135. https://doi.org/10.3109/01677063.2013.856430
Luo J, Bavencoffe A, Yang P, Feng J, Yin S, Qian A, Hu H (2018) Zinc inhibits TRPV1 to alleviate chemotherapy-induced neuropathic pain. J Neurosci 38(2):474–483
Maihöfner C, Diel I, Tesch H, Quandel T, Baron R (2021) Chemotherapy-induced peripheral neuropathy (CIPN): current therapies and topical treatment option with high-concentration capsaicin. Support Care Cancer 29(8):4223–4238. https://doi.org/10.1007/s00520-021-06042-x
Majithia N, Loprinzi CL, Smith TJ (2016) New practical approaches to chemotherapy-ınduced neuropathic pain: prevention, assessment, and treatment. Oncology-New York 30(11):1020–1029
Micheli L, Di Cesare Mannelli L, Del Bello F, Giannella M, Piergentili A, Quaglia W, Ghelardini C (2020) The use of the selective imidazoline I1 receptor agonist carbophenyline as a strategy for neuropathic pain relief: preclinical evaluation in a mouse model of oxaliplatin-induced neurotoxicity. Neurotherapeutics 17(3):1005–1015
Nadipelly J, Sayeli V, Kadhirvelu P, Shanmugasundaram J, Cheriyan BV, Subramanian V (2018) Effect of certain trimethoxy flavones on paclitaxel-induced peripheral neuropathy in mice. Integr Med Res 7(2):159–167
Neugebauer V, Han JS, Adwanikar H, Fu Y, Ji G (2007) Techniques for assessing knee joint pain in arthritis. Mol Pain 3:1744–8069
Obata K (2005) TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Investig 115(9):2393–2401. https://doi.org/10.1172/JCI25437
Omran M, Belcher EK, Mohile NA, Kesler SR, Janelsins MC, Hohmann AG, Kleckner IR (2021) Review of the role of the brain in chemotherapy-ınduced peripheral neuropathy. Front Mol Biosci 8:693133. https://doi.org/10.3389/fmolb.2021.693133
Pareek (2011) Feverfew (Tanacetum parthenium 535 L.): a systematic review. Pharmacogenet Rev 5:103–110
Patil CS, Singh VP, Satyanarayan PSV, Jain NK, Singh A, Kulkarni SK (2003) Protective effect of flavonoids against aging-and lipopolysaccharide-induced cognitive impairment in mice. Pharmacology 69(2):59–67
Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, Jegla T, Patapoutian A (2007) A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological ınhibition. Mol Pain 3:1744-8069-3-40. https://doi.org/10.1186/1744-8069-3-40
Pittler (2004) Feverfew or preventing migraine. Cochrane Database Syst 537 Rev 1 CD002286
Polomano RC, Bennett GJ (2001) Chemotherapy-evoked painful peripheral neuropathy. Pain Med 2(1):8–14. https://doi.org/10.1046/j.1526-4637.2001.002001008.x
Privitera R, Anand P (2021) Capsaicin 8% patch Qutenza and other current treatments for neuropathic pain in chemotherapy-induced peripheral neuropathy (CIPN). Curr Opin Support Palliat Care 15(2):125–131. https://doi.org/10.1097/SPC.0000000000000545
Salat K (2020) Chemotherapy-induced peripheral neuropathy: part 1-current state of knowledge and perspectives for pharmacotherapy. Pharmacol Rep 72(3):486–507. https://doi.org/10.1007/s43440-020-00109-y
Salehifar E, Janbabaei G, Hendouei N, Alipour A, Tabrizi N, Avan R (2020) Comparison of the efficacy and safety of pregabalin and duloxetine in taxane-ınduced sensory neuropathy: a randomized controlled trial. Clin Drug Investig 40(3):249–257. https://doi.org/10.1007/s40261-019-00882-6
Saranitzky (2009) Feverfew for 544 migraine prophylaxis: a systematic review. J Diet Suppl 6:91–103
Smith EML, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C, Le-Lindqwister N, Gilman PB, Shapiro CL, Alliance for Clinical Trials in Oncology (2013) Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA Oncol 309(13):1359–1367. https://doi.org/10.1001/jama.2013.2813
Son DB, Choi W, Kim M, Go EJ, Jeong D, Park CK, Kim YH, Lee H, Suh JW (2021) Decursin alleviates mechanical allodynia in a paclitaxel-ınduced neuropathic pain mouse model. Cells 10(3). ARTN 547. https://doi.org/10.3390/cells10030547
Souza Monteiro de Araujo D, Nassini R, Geppetti P, De Logu F (2020) TRPA1 as a therapeutic target for nociceptive pain. Expert Opin Ther Targets 24(10):997–1008. https://doi.org/10.1080/14728222.2020.1815191
Staaf S, Oerther S, Lucas G, Mattsson JP, Ernfors P (2009) Differential regulation of TRP channels in a rat model of neuropathic pain. Pain 144(1):187–199. https://doi.org/10.1016/j.pain.2009.04.013
Summers MN, Haley WE, Reveille JD, Alarcon GS (1988) Radiographic assessment and psychologic variables as predictors of pain and functional ımpairment in osteo-arthritis of the knee or hip. Arthritis Rheum 31(2):204–209. https://doi.org/10.1002/art.1780310208
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, von Mering C (2019) STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47(D1):D607–D613. https://doi.org/10.1093/nar/gky1131
Testa (1996) Assessment of qality-of-life outcomes. N Engl J Med 334(13):835–840
Tsantoulas C, Zhu L, Shaifta Y, Grist J, Ward JP, Raouf R, McMahon SB (2012) Sensory neuron downregulation of the Kv9. 1 potassium channel subunit mediates neuropathic pain following nerve injury. J Neurosci 32(48):17502–17513
Tsantoulas C, Denk F, Signore M, Nassar MA, Futai K, McMahon SB (2018) Mice lacking Kcns1 in Peripheral neurons show ıncreased basal and neuropathic pain sensitivity. Pain 159(8):1641–1651. https://doi.org/10.1097/j.pain.0000000000001255
Uchi (2002) The sesquiterpene lactone parthenolide inhibits LPS- but not TNFalpha- induced maturation of human monocyte-derived dendritic cells by inhibition of the p38 mitogen-activated protein kinase pathway. J Allergy Clin Immunol 110(2):269–276
Velasco-González R, Coffeen U (2022) Neurophysiopathological aspects of paclitaxel-induced peripheral neuropathy. Neurotox Res 40(6):1673–1689. https://doi.org/10.1007/s12640-022-00582-8
Wheeler DW, Lee MC, Harrison EK, Menon DK, Woods CG (2014) Case Report: Neuropathic pain in a patient with congenital insensitivity to pain. F1000Research 26;3:135. https://doi.org/10.3389/fmolb.2021.693133
WHO (2007) Normative Guidelines On Pain Management. Report of a Delphi Study to Determine the Need for Guidelines and to Identify the Number and Topics of Guidelines That Should Bedeveloped by WHO. Report Prepared by Prof Neeta Kumar, Consultant. Geneva
Woolf (1999) Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 353(9168):1959–64
Woolf (2004) American College of Physicians; American Physiological Society. Pain: Moving from Symptom Control toward Mechanism-Specific Pharmacologic Management. Ann Intern Med 140(6):441–51
Xue Y, Chidiac C, Herault Y, Gaveriaux-Ruff C (2021) Pain behavior in SCN9A (Nav1. 7) and SCN10A (Nav1. 8) mutant rodent models. Neurosci Lett 753:135844
Yang S, Chang MC (2019) Chronic pain: structural and functional changes in brain structures and associated negative affective states. Int J Mol Sci 20(13). ARTN 3130. https://doi.org/10.3390/ijms20133130
Yesilkent EN, Ceylan H (2022) Investigation of the multi-targeted protection potential of tannic acid against doxorubicin-induced kidney damage in rats. Chem-Biol Interact 365:110111. https://doi.org/10.1016/j.cbi.2022.110111
Zhang J, Rong L, Shao J, Zhang Y, Liu Y, Zhao S, Cao J (2021) Epigenetic restoration of voltage‐gated potassium channel Kv1. 2 alleviates nerve injury‐induced neuropathic pain. J Neurochem 156(3):367–378
Zhang YL, Zhang DB, Li WQ, Chen JQ, Peng YF, Cao W (2003) A novel real-time quantitative PCR method using attached universal template probe. Nucleic Acids Res 31(20). ARTN e123. https://doi.org/10.1093/nar/gng123
Zhou Y, Liu D, Chen S, Chen N, Sun J, Wang X, Cao F, Tian Y, Ye D (2020) Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain. Acta Pharmacol Sin 41(8):1041–1048. https://doi.org/10.1038/s41401-020-0394-6
Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16(2):109–110. https://doi.org/10.1016/0304-3959(83)90201-4
Funding
Financial support for the present research was received from Atatürk University Scientific Research Projects Coordination Commission [Grant Number: FDK-2020-8345].
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: HB (group leader), ET and AH. Performed the experiments: ET, CB, SS and MÖ. Analyzed the data: ET, CB and MÖ. Contributing reagents/materials/analysis tools: HB. Wrote the paper: HB, ET and MÖ. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
With the decision no. 193 of Atatürk University Animal Experiments Local Ethics Committee (AUHADYEK) in its session dated 07.11.2019 and numbered 14, all stages of our study were approved to comply with ethical rules.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Toraman, E., Bayram, C., Sezen, S. et al. Parthenolide as a potential analgesic in the treatment of paclitaxel-induced neuropathic pain: the rat modeling. Naunyn-Schmiedeberg's Arch Pharmacol 396, 3707–3721 (2023). https://doi.org/10.1007/s00210-023-02568-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02568-5