Abstract
Acute respiratory distress syndrome (ARDS) is a serious intensive care condition. Despite advances in treatment over the previous few decades, ARDS patients still have high fatality rates. Thus, more research is needed to improve the outcomes for people with ARDS. Minocycline is an antibiotic with antioxidant, anti-inflammatory, and anti-apoptotic effects. In the current investigation, the therapeutic effects of minocycline on oleic acid-induced ARDS were evaluated. Male rats were classified into 6 groups, 1. control (normal saline), 2. oleic acid (100 µL, i.v.), 3–5. oleic acid + minocycline (50, 100, 200 mg/kg, i.p.), and 6. minocycline (200 mg/kg, i.p.) alone. Twenty-four hours after the oleic acid injection, the lung tissue is isolated, weighed, and the middle part of the right lung is immediately placed in the freezer, while the middle part of the left lung is placed in formalin and sent to the laboratory for pathology testing. Then, the amounts of malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), cytokines (interleukin-1 beta (IL-1β), tumor necrosis factor-α (TNF-α)), B-cell lymphoma 2 (Bcl-2), Bcl-2 associated X (Bax), and cleaved caspase-3 were determined in lung tissue. Administration of oleic acid increased emphysema, inflammation, vascular congestion, hemorrhage, MDA amount, Bax/Bcl-2 ratio, cleaved caspase-3, IL-1β, TNF-α levels, and decreased GSH, SOD, and CAT levels in comparison with the control group. The administration of minocycline could significantly reduce pathological and biochemical alterations induced by oleic acid. Minocycline has a therapeutic effect on oleic acid-induced ARDS through antioxidant, anti-inflammatory, and anti-apoptotic properties.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute respiratory distress syndrome (ARDS) is characterized by acute hypoxia, respiratory failure, and alveolar damage as a result of a strong pulmonary inflammatory response triggered by systemic or local insults that impair the alveolar-capillary structure, resulting in amplified permeability in capillaries (Akella et al. 2014; Gonçalves-de-Albuquerque et al. 2015). The impairment in gas exchange, the heterogeneous collapse of air sacs with a prevalence in the dependent regions, and pulmonary inflammatory edema are all ARDS symptoms (Borges et al. 2019). The mortality rate of ARDS has not been lowered to less than 30% with scientific clinical care. Although research into the pathophysiology and therapy of ARDS has continued to advance since 1967, ARDS has remained a severe clinical challenge, owing to a lack of greater knowledge of the disease pathomechanism. The major cell death mechanisms in ARDS are apoptosis and necrosis (Pan et al. 2016). Furthermore, oxidative stress and inflammatory responses are thought to influence the development of ARDS. Cytokines and pro-inflammatory mediators in the bronchoalveolar lavage fluid or serum reliably indicate activation of polymorphonuclear leukocytes, monocytes, and macrophages in the bronchoalveolar lavage fluid or serum (Bhatia and Moochhala 2004; Liu et al. 2015). There are currently no effective and specific drugs for ARDS. As a result, it appears that searching for effective therapeutic agents for the treatment of ARDS is necessary.
Animal models of lung injury have been created to imitate human ARDS and find out the underlying mechanisms of this disorder. Unluckily, no animal model perfectly replicates human ARDS. Animal studies, on the other hand, can provide essential information on the physiopathology of lung injury in humans, and in this case, they could serve as a probable connection from bench to bedside (Gonçalves-de-Albuquerque et al. 2015). In line with the early stages of ARDS, the oleic acid, an unsaturated fatty acid in animals and plants, model generates abnormalities in capillary permeability, as well as impairments in pulmonary mechanics and gas exchange (Matute-Bello et al. 2008). Oleic acid causes a morphologically heterogeneous lung lesion, similar to clinical ARDS, with areas of mild pulmonary damage contrasted with areas of severe destruction (Ballard-Croft et al. 2012). In the early stages of oleic acid-induced lung injury, several alterations occur including morphological changes, alveolar epithelial cell necrosis, interstitial/intra-alveolar edema, severe capillary congestion, and both cell types standing out from their basal membranes (Borges et al. 2019).
Minocycline is a semi-synthetic tetracycline analog that has broad-spectrum activity against several spirochetes, Plasmodium spp., Chlamydia, Mycoplasma pneumoniae, Rickettsia, and Gram-negative and Gram-positive bacteria (Garrido‐Mesa et al. 2013, Singh et al. 2020). Since 1972, this antibiotic has been used to treat rheumatoid arthritis and infections caused by susceptible micro-organisms (Ochsendorf 2010). Several research projects in recent years have validated its non-antibiotic characteristics, comprising anti-cancer, neuroprotective, anti-apoptotic, antioxidant, and anti-inflammatory effects (Garrido‐Mesa et al. 2013). It has also been reported that the anti-inflammatory and immunomodulatory properties of minocycline may be beneficial in the treatment of coronavirus disease 2019 (COVID-19) patients, particularly in the case of respiratory problems such as ARDS and multiorgan damage (Singh et al. 2020). It was observed that minocycline inhibited oxidative stress and inflammation in sepsis-induced acute lung injury (Cui et al. 2021). Minocycline has been demonstrated to inhibit the emission of cytokines such as interleukin-6 (IL-6), IL-2, and tumor necrosis factor-alpha (TNF-α), suggesting that it may be useful in treating disease-related cytokine release syndrome (Seabrook et al. 2006; Szeto et al. 2010, Garrido‐Mesa et al. 2013). Moreover, it has been reported that minocycline prevented cell death by blocking apoptotic cascades and decreasing the amount of caspases-1 and 3 (Garrido‐Mesa et al. 2013).
Consequently, considering the significant roles of oxidative damage, inflammation, and apoptosis in ARDS and the fact that minocycline increases radical scavenging, and inhibits inflammation and apoptosis, the present investigation was designed to examine the ameliorative properties of minocycline in oleic acid-induced ARDS in the rat by measuring oxidative stress (the amount of malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), catalase (CAT)), inflammation (TNF-α and IL-1β amounts), and apoptosis (B-cell lymphoma 2 (Bcl-2), Bcl-2 associated X (Bax), and cleaved caspase-3) in lung tissue.
Materials and methods
Animals
The Animal Center, School of Pharmacy, Mashhad University of Medical Sciences, Iran, provided the male Wistar rats (250–300 g). Animals were chosen at random and housed in conventional cages in the animal room at Mashhad University of Medical Sciences, Iran, on a switching 12-h light-dark cycle at ambient temperature (25 ± 2 °C) and relative humidity (50 ± 10%). The animals were fed a normal diet and allowed free access to water and food.
The Ethical Committee Acts of Mashhad University of Medical Sciences accepted all evaluations for the care and use of experimental animals (IR.MUMS.PHARMACY.REC.1399.066). For a week before the start of the experiments, the rats were allowed to adapt to their new surroundings.
Chemicals
Oleic acid, tween 20, sodium fluoride, sodium orthovanadate, β-glycerol phosphate, sodium deoxycholate, phenylmethanesulfonyl fluoride (PMSF), and bromophenol blue were purchased from Sigma Aldrich. Ethyl alcohol, potassium chloride, phosphoric acid, 2-thiobarbituric acid (TBA), n-butanol, 5,5-dithio-bis-2-nitrobenzoic acid (DTNB), Tris–HCl, ethylenediaminetetraacetic acid (EDTA), ethylene glycol tetraacetic acid (EGTA), 2-mercaptoethanol (2-ME), sodium dodecyl sulfate (SDS), glycerol, dry skim milk, sodium hydroxide, di-sodium hydrogen phosphate dihydrate, sodium phosphate monobasic monohydrate, trichloroacetic acid 10% (TCA 10%), sodium citrate 10%, Thiazolyl blue tetrazolium bromide (MTT), pyrogallol, dimethyl sulfoxide (DMSO), and hydrogen peroxide were bought from Merck, Germany. A protease inhibitor cocktail and Pierce enhanced chemiluminescence (ECL) Western blotting substrate were obtained from Thermo Fisher Scientific. Polyvinylidene difluoride (PVDF) was purchased from Bio-Rad. Methanol and n-butanol were purchased from the Industrial Chemical Complex Company Laboratory and pharmaceutical chemicals by Dr Mojallali, Tehran, Iran.
Study groups
The rats were randomly divided into six groups of six animals each and subjected to the following treatments:
-
1. Control: the animals in this group received 100 µL of intravenous normal saline and then minocycline solvent (normal saline, 300 µL) intraperitoneally.
-
2. Oleic acid group: the animals in this group received 100 µL of intravenous oleic acid and minocycline solvent (normal saline, 300 µL) intraperitoneally.
-
3–5. The animals in these groups received 100 µL of intravenous oleic acid and minocycline 50, 100, and 200 mg/kg, intraperitoneally 30 min after the oleic acid injection (Cui et al. 2021).
-
6. The animals in this group received minocycline (200 mg/kg, i.p.).
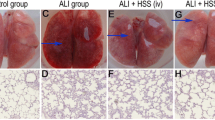
Twenty-four hours after oleic acid injection, the lung tissue was isolated, weighed, and the middle part of the right lung was immediately placed in the freezer, and the middle part of the left lung was placed in formalin and sent to the laboratory for pathology testing. It is also noteworthy to note that the histopathological alterations besides GSH and MDA levels were the criteria for lung injury.
Immunohistochemistry analysis with hematoxylin and eosin (H&E) staining
Lung tissues were isolated and treated with 10% formalin shortly after the rats were sacrificed. The specimens were then dehydrated and embedded in paraffin wax before being cut into 4 mm pieces and stained with H&E. Our team planned a grading system to assess pulmonary architecture, leukocyte infiltration, and lung edema formation with a subjective scoring on a scale of 1 to 4, with 1 signifying normal and 4 indicating the most serious harm.
Determination of lipid peroxidation in the lung tissue
The level of MDA, a lipid peroxidation marker, was measured in tissue homogenates using a previously reported method (Rahbardar et al. 2022). In cold potassium chloride, a 10% w/v homogenate of rat lung was made (1.15%). A mixture of 0.5 mL homogenized lung, 3 mL phosphoric acid 1%, and 1 mL 0.6% TBA was prepared. For 45 min, the tubes were immersed in a boiling water bath (95 °C). A total of 4 mL n-butanol was added to the tubes after they had cooled, and they were vortexed for 1 min. The tubes were centrifuged for 20 min at 3000 g. The absorbance of the upper layer was then measured at 532 nm wavelengths. The MDA concentration was estimated using a standard curve with a concentration range of 0–100 nmol/mL (Uchiyama and Mihara 1978).
Determination of GSH level in the lung tissue
Free sulfhydryl groups in lung tissue react with DTNB reagent to generate a yellow color, according to the Moron et al. method (Moron et al. 1979). For this, 200 mg of lung tissue was combined with 1:1 V/V of TCA (10%) in phosphate buffer (pH = 7.4) and centrifuged at 3000 g for 10 min. The supernatant was then collected and combined with 0.5 mL DTNB and 2.5 mL phosphate buffer (pH = 8). Finally, the UV-spectrophotometer (Jenway 6105 UV/Vis, UK) was used to measure absorbance at 412 nm. The results were expressed in nmol/g tissue (Eisvand et al. 2022).
Determination of SOD level in the lung tissue
The activity of SOD was measured using a colorimetric technique. SOD is produced by auto-oxidation of pyrogallol and is reliant on MTT to formazan suppression. The reaction is suppressed after adding DMSO, which acts as a color stabilizer. Briefly, homogenized lung tissue was placed in wells of a microplate containing buffer and MTT, followed by the addition of pyrogallol and incubation at room temperature (5 min). In a microplate reader, it was read at 570 nm and 630 nm when DMSO was added. One unit of SOD is required to prevent a 50% drop in MTT. The results were given in units/g tissue (Bargi et al. 2017).
Determination of CAT level in the lung tissue
The decomposition of hydrogen peroxide was used to assess catalase activity, which was measured by a decrease in absorbance at 240 nm. For this, two distinct solutions were prepared: a 30 mM hydrogen peroxide solution as a substrate, and a 50 mM phosphate buffer (pH = 7) in a solution blank as an alternate substrate. Hydrogen peroxide and lung tissue homogenates were present in sufficient concentrations in the sample solutions. Hydrogen peroxide was added, and the reduction in absorption was monitored using a spectrophotometer at 240 nm as the reaction progressed (Bargi et al. 2017).
Western blot analysis
The protein extract was subjected to Western blot analysis to assess the changes in apoptosis and inflammatory markers. Unfrozen lung tissue (100 mg) was homogenized in a lysis buffer comprising 50 mM Tris–HCl (pH 7.4), 2 mM EDTA, 2 mM EGTA, 10 mM sodium fluoride, 1 mM sodium orthovanadate (2H2O), 10 mM β-glycerophosphate, 0.2% W/V sodium deoxycholate and, 1 mM PMSF, and complete protease inhibitor cocktail (Roche, Mannheim, Germany). On the ice, the homogenate was sonicated for 30 s at 10-s intervals. It was centrifuged at 4 °C for 10 min at 10,000 g. The supernatants were then separated and the protein content of the supernatants was determined using the Bradford technique (Ghasemzadeh Rahbardar et al. 2020). The samples were then boiled after being combined 1:1 with 2XSDS blue buffer (5 min). After cooling, the samples were stored in an –80 °C freezer until they were analyzed. Samples were electrophoresed on a 12% SDS polyacrylamide gel, transferred to PVDF paper, and blocked for 2 h at room temperature in 5% non-fat milk powder (skimmed milk) in Tris-buffered saline tween 20 (TBST). Primary antibodies included rabbit polyclonal anti-serum against Bax (Cell Signaling, #2772), rabbit monoclonal anti-serum against Bcl-2 (Cell Signaling, #2870), rabbit monoclonal anti-serum against cleaved caspase-3 (Cell Signaling, #9664), rabbit polyclonal anti-serum against TNF-α (Cell Signaling, #3707), rabbit anti-IL-1β (Abcam #9722), and mouse monoclonal anti-serum against β-actin (Cell Signaling, #3700) at 1:1000 dilutions. After the PVDF papers were washed three times with TBST for 5 min each, the membranes were incubated for 1.5 h at room temperature with anti-rabbit IgG labeled with horseradish peroxidase (Cell Signaling, #7074) or anti-mouse IgG labeled with horseradish peroxidase (Cell Signaling, #7076) at 1:3000 dilutions. Increased chemiluminescence revealed the peroxidase-coated protein bands. The integrated optical densities of the bands were measured using the Alliance 4.7 Gel doc (UK). Protein band densitometric analysis was performed using UV Tec Software (UK). To standardize the protein levels, β-actin was employed as a control protein.
Statistical analysis
The quantitative data were presented as a mean ± standard deviation (mean ± SD). GraphPad Prism 8.0 (GraphPad Prism Software Inc., San Diego, CA, USA) was used to conduct the statistical analysis. Tukey’s multiple comparison test or the Kruskal–Wallis test followed by Dunn’s multiple comparisons were used to analyze the numerous groups in a one-way analysis of variance. Statistical significance was defined as a P value of less than 0.05. For the behavioral assessments, data were reported as medians with interquartile ranges for each group, and statistical analysis was performed using the Kruskal–Wallis nonparametric test and Dunn’s multiple comparison.
Results
The effect of minocycline on oleic acid-induced histopathological alterations in lung tissue
The administration of oleic acid caused histopathological changes in rats’ lungs, for instance, emphysema (P < 0.01), inflammation (P < 0.001), vascular congestion, and hemorrhage (P < 0.05) in comparison with the control group. Although concurrent treatment with minocycline (50, 100, and 200 mg/kg) along with oleic acid could not decrease emphysema, it significantly reduced inflammation (P < 0.001), vascular congestion, and hemorrhage (P < 0.05) in comparison with the oleic acid group (Fig. 1). Receiving minocycline (200 mg/kg) alone had no significant effect on lung tissue versus the control group (Table 1).
The effect of minocycline on the amount of MDA and GSH
Lipid peroxidation was demonstrated in the oleic acid (100 µL) group by an increase in MDA levels in the lung tissue (P < 0.05) in comparison with the control group. MDA levels were significantly reduced when 50 mg/kg (P < 0.01), 100 mg/kg (P < 0.01), and 200 (P < 0.05) mg/kg minocycline were administered with oleic acid compared to oleic acid. When comparing the minocycline (200 mg/kg) alone group to the control group, there was no significant change (Fig. 2A).
Effect of minocycline on oleic acid-induced A lipid peroxidation and B GSH level in lung tissue. The data is provided as a mean ± SD (n = 6). An analysis of variance (ANOVA) was used, followed by a post hoc analysis. For statistical analysis, Tukey’s test was utilized. ###P < 0.001, #P < 0.05 when compared with the control group, ***P < 0.001, **P < 0.01, and *P < 0.05 when compared to oleic acid group. GSH, glutathione; MDA, malondialdehyde; Mino, minocycline; OA, oleic acid
GSH levels were considerably lower in the oleic acid (100 µL) group compared to the control group (P < 0.001), as seen in Fig. 2B. In comparison to the oleic acid group, minocycline (50, 100 mg/kg) was able to enhance GSH levels (P < 0.001). However, the administration of minocycline 200 mg/kg along with oleic acid could not increase the GSH amount compared to the oleic acid group. There was no significant difference between the control and minocycline (200 mg/kg) alone groups.
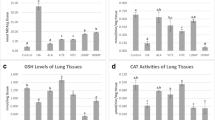
The effect of minocycline on the amount of SOD and CAT
The results in Fig. 3A show that oleic acid (100 µL) intravenous injection significantly lowered the SOD amount when compared to the control group (P < 0.001). Intraperitoneal injection of minocycline 50 mg/kg (P < 0.01), 100 mg/kg (P < 0.001), and 200 mg/kg (P < 0.01) along with oleic acid significantly boosted SOD enzyme level as compared to the oleic acid group. The results also showed that the administration of minocycline (200 mg/kg) alone to rats had no significant effect on SOD content in comparison with the control group.
Effect of minocycline on oleic acid-induced A SOD and B CAT levels in lung tissue. The data is provided as a mean ± SD (n = 6). An analysis of variance (ANOVA) was used, followed by a post hoc analysis. For statistical analysis, Tukey’s test was utilized. ###P < 0.001 when compared to the control group, ***P < 0.001, **P < 0.01, and *P < 0.05 when compared with the oleic acid group. CAT, catalase; Mino, minocycline; OA, oleic acid; SOD, superoxide dismutase
The CAT amount in the lung tissues of the oleic acid (100 µL) group was considerably lower than the control group (P < 0.001), as shown in Fig. 3B. Minocycline (50 and 100 mg/kg) was found to significantly boost CAT levels when administered with oleic acid in comparison with the oleic acid group (P < 0.05). However, concurrent prescription of minocycline 200 mg/kg with oleic acid had no significant effect on the CAT amount compared to the oleic acid group. In comparison to the control rodents, minocycline 200 mg/kg alone did not affect the CAT level.
The effect of minocycline on the amount of IL-1β and TNF-α
The most efficient dose of minocycline, minocycline 50 mg/kg, was determined for Western blot biochemical tests based on the results of histopathological and oxidative stress experiments.
When the animals received oleic acid (100 µL), the amount of IL-1β increased (P < 0.001) compared to the control group. Co-administration of minocycline and oleic acid reduced IL-1β levels in comparison to the oleic acid group (P < 0.001). Injection of oleic acid (200 mg/kg) alone did not affect the IL-1β level when compared to the control group (Fig. 4A, B).
Effect of oleic acid and minocycline on the IL-1β and TNF-α levels in the lung tissue. A Specific bands for IL-1β and TNF-α levels have been investigated using Western blot. B IL-1β level data by densitometric analysis. C TNF-α level data by densitometric analysis. The data is mean ± SD (n = 5). ANOVA and posttest Tukey–Kramer were used for statistical analysis. ###P < 0.001 compared with the control group, and ***P < 0.001 and **P < 0.01 compared with the oleic acid group. Mino, minocycline; OA, oleic acid
In comparison to the control group, the level of TNF-α increased (P < 0.001) when the animals were administered 100 µL of oleic acid. In comparison to the oleic acid group, minocycline and oleic acid co-administration lowered TNF-α levels (P < 0.01). When compared to the control group, injections of oleic acid (200 mg/kg) did not affect TNF-α amounts (Fig. 4A, C).
The effect of minocycline on the amount of Bax/Bcl-2 ratio and cleaved caspase-3
The administration of oleic acid (100 µL) resulted in an increased Bax/Bcl-2 ratio compared with the animals in the control group (P < 0.01). Concurrent administration of minocycline and oleic acid reduced the Bax/Bcl-2 ratio in comparison with the oleic acid group (P < 0.05). Receiving oleic acid (200 mg/kg) alone caused no considerable alteration in the Bax/Bcl-2 ratio vs. the control group (Fig. 5A, B).
Effect of oleic acid and minocycline on the Bax/Bcl-2 ratio and cleaved caspase-3 level in the lung tissue. A Specific bands for Bax/Bcl2 ratio and cleaved caspase-3 level have been investigated using Western blot. B Bax/Bcl-2 ratio data by densitometric analysis. C Cleaved caspase-3 level data by densitometric analysis. The data is mean ± SD (n = 5). ANOVA and posttest Tukey–Kramer were used for statistical analysis. ###P < 0.001, ##P < 0.01 compared with the control group, and *P < 0.05 compared with the oleic acid group. Mino, minocycline; OA, oleic acid
When oleic acid (100 µL) was injected into the animals, the cleaved caspase-3 amount rose (P < 0.001) when compared to the control group. In comparison to the oleic acid group, concurrent administration of minocycline and oleic acid lowered the cleaved caspase-3 level (P < 0.05). In comparison to the control group, receiving oleic acid (200 mg/kg) alone had no significant effect on the level of cleaved caspase-3 (Fig. 5A, C).
Discussion
The purpose of this study was to investigate if minocycline could ameliorate ARDS as a result of oleic acid-induced elevated oxidative stress, inflammation, and apoptosis. The data revealed that intravenous injection of 100 µL oleic acid caused significant emphysema, inflammation, vascular congestion, and hemorrhage in lung tissue in comparison with the control group. It also increased MDA amount and decreased GSH, SOD, and CAT levels in lung tissue. Moreover, oleic acid augmented the Bax/Bcl-2 ratio, cleaved caspase-3, TNF-α, and IL-1β amounts. On the other hand, the administration of minocycline (50, 100, 200 mg/kg) intraperitoneally ameliorated inflammation, vascular congestion, and hemorrhage in pathological tests. However, they did not affect oleic acid-induced emphysema. Minocycline (50, 100, 200 mg/kg) concurrent administration along with oleic acid could reduce oxidative stress by decreasing MDA and increasing GSH, SOD, and CAT levels, too. Moreover, minocycline 50 mg/kg significantly lowered Bax/Bcl-2 ratio, cleaved caspase-3, TNF-α, and IL-1β levels in lung tissue compared with the oleic acid group. It is also noteworthy to mention that administration of minocycline 200 mg/kg alone caused no considerable changes in the studied parameters in comparison with the control group.
The key pathologic characteristics of ARDS include surfactant inactivation, protein-rich pulmonary edema, and disruptions of the alveolar-capillary barrier (Chen et al. 2018). As a result, endothelial cell repair or regeneration may have an unanticipated therapeutic effect on ARDS. The pathological analysis of the current study disclosed that administration of oleic acid (100 µL, i.v.) to male rats caused considerable emphysema, inflammation, vascular congestion, and hemorrhage in lung tissue in comparison with the control rats. In line with our findings, a previous investigation reported that administration of oleic acid (50 µL, i.v.) to rats resulted in intra-alveolar edema, increased presence of macrophages in intra-alveolar locations, and a significant rise in neutrophil counts in intra-alveolar and interstitial areas, notably surrounding the congestive capillaries (Erdem et al. 2017). Another study has also stated that receiving oleic acid (by continuous infusion of 0.2 mL/kg) caused inflammation, edema, hemorrhage, septal necrosis, and collapsed alveoli in the lung tissue of large white pigs (Borges et al. 2019). In our work, it was observed that co-administration of minocycline (50, 100, 200 mg/kg, i.v.) with oleic acid reduced inflammation, vascular congestion, and hemorrhage. The results of a study indicated that the administration of minocycline (15 mg/kg, 28 days, i.p.) to mice with methotrexate-induced pulmonary fibrosis reversed pathological alterations in lung tissue (Kalemci et al. 2013).
According to clinical and experimental findings, oxidative stress is implicated in the pathology of acute lung injury. Excessive production of reactive oxygen species (ROS) under oxidative stress damages cellular membranes via lipid peroxidation, which increases vascular permeability and leads to serum protein leakage into the alveoli (Li et al. 2021). The results of our study indicated that intravenous injection of oleic acid amplified MDA levels, and reduced GSH, SOD, and CAT amounts in lung tissue. The administration of oleic acid (0.1 mL/kg) to mice in Li et al. study also increased MDA and decreased SOD amount in comparison to the control group (Li et al. 2021). Moreover, chemical colorimetry assays of another study revealed that oleic acid-induced acute lung injury had increased ROS generation and decreased antioxidant capacity in rats (Chen et al. 2015). In the current study, concurrent administration of minocycline with oleic acid lowered MDA amount and augmented GSH, SOD, and CAT levels in lung tissue. In line with our findings, the effectiveness of minocycline (3 and 15 mg/kg, 28 days, i.p.) in mice with methotrexate-induced lung fibrosis was also illustrated. Minocycline could reduce oxidative stress that attenuated MDA levels and increased CAT amount in lung tissue (Kalemci et al. 2013). Furthermore, a recent study reported that oral administration of minocycline (10, 20, and 40 mg/kg, 7 days, p.o.) decreased MDA amount and increased SOD, CAT, and GSH levels in lung tissue of mice exposed to hypoxia (Eduviere and Otomewo 2022).
Several investigations have shown that ROS stimulates innate immune cell activation and the consequent release of inflammatory cytokines for instance IL-6 and TNF-α, proposing that ROS, in conjunction with inflammatory cytokines, might induce vascular endothelial dysfunction and promote the progression of acute lung injury, including ARDS (Porter and Hall 2009; Li et al. 2021). In the current study, it was observed that administration of oleic acid caused an upsurge in the levels of IL-1β and TNF-α in lung tissue in comparison with the control group. In agreement with our data, another investigation revealed that the administration of oleic acid in an acute lung injury model increased IL-6 and IL-8 levels in comparison with the healthy large white pigs (Borges et al. 2019). Our obtained findings disclosed that administration of minocycline along with oleic acid reduced IL-1β and TNF-α amounts in lung tissue compared to the rats in the oleic acid group. In line with our findings, it was reported that minocycline decreases inflammatory and oxidative injury in a septic lung injury model induced intestinal perforation by down-regulating the expressions of TNF-α, IL-6, IL-1β, and MDA in lung tissues of the mice (Cui et al. 2021).
ARDS-induced pulmonary injury, inflammation, and oxidative stress are all linked to apoptosis (Mokra et al. 2016). Caspase-3 has been linked to the start of the “death cascade” and is thus an important marker of the cells’ entry point into the apoptotic signaling pathway (Borges et al. 2019). Our results also have shown that receiving oleic acid-induced apoptosis in lung tissue by increasing Bax/Bcl-2 ratio and caspase-3 levels in comparison with the control group. In line with our results, it has been reported that the anti-apoptotic marker Bcl-2 was reduced by oleic acid (0.9 mL/kg, i.v.), but the pro-apoptotic marker Bcl-2 associated agonist of cell death (Bad) was significantly increased (Guo et al. 2011). Another study has also disclosed that exposure to oleic acid elevated caspase-3 amounts in A549 cells (Chen et al. 2015). In our research, the administration of minocycline reversed the alterations induced by oleic acid in apoptotic and anti-apoptotic factors. The anti-apoptotic property of minocycline was disclosed in a rat model of ischemic renal injury (Kelly et al. 2004) and also on PC12 cells against cadmium-induced neurotoxicity (Shayan et al. 2022) by reducing the amount of apoptotic markers.
In general, it can be stated that all three doses of minocycline were effective in reducing the effects of oleic acid in inducing ARDS. But lower doses performed better in some tests, including CAT, SOD, and GSH levels, although there may not be statistically significant differences among doses for some tests. The fact that lower doses of drugs are more successful can be justified by the fact that some chemicals and drugs induce oxidative stress (prooxidant) and apoptosis in higher doses.
Limitations of the present study
-
Only male rats were employed in our investigation. With strong and detectable differences in more than half of the genes’ expression patterns between males and females, sex differences in preclinical models are becoming more and more obvious. Hence, female animal models should be used in upcoming research.
-
We did not evaluate physiological data such as gas exchange because measuring them was difficult due to the small size of the animals.
Conclusion
In this study, we investigated the underlying mechanism of oleic acid-induced ARDS and examined the potential protection of minocycline against this disorder. Concluding from our observations, oleic acid-induced oxidative stress and inflammation ultimately lead to apoptotic cell death. However, concurrent administration of oleic acid and minocycline to rats inhibited oxidative stress (by decreasing MDA amount and increasing GSH, SOD, and CAT), inflammation (by attenuating IL-1β and TNF-α), and apoptosis (through lowering Bax/Bcl-2 ratio and cleaved caspase-3 level). Therefore, minocycline might be a promising agent in treating and managing ARDS.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- 2-ME:
-
2-Mercaptoethanol
- ARDS:
-
Acute respiratory distress syndrome
- Bad:
-
Bcl-2 associated agonist of cell death
- Bax:
-
Bcl-2 associated X
- Bcl-2:
-
B-cell lymphoma 2
- CAT:
-
Catalase
- COVID-19:
-
Coronavirus disease 2019
- DMSO:
-
Dimethyl sulfoxide
- DTNB:
-
5,5-Dithio-bis-2-nitrobenzoic acid
- EDTA:
-
Ethylenediaminetetraacetic acid
- EGTA:
-
Ethylene glycol tetraacetic acid
- GSH:
-
Glutathione
- H&E:
-
Hematoxylin and eosin
- i.p.:
-
Intraperitoneal
- IL-6:
-
Interleukin-6
- MDA:
-
Malondialdehyde
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
- PMSF:
-
Phenylmethanesulfonyl fluoride
- PVDF:
-
Polyvinylidene difluoride
- ROS:
-
Reactive oxygen species
- SDS:
-
Sodium dodecyl sulfate
- SOD:
-
Superoxide dismutase
- TBA:
-
2-Thiobarbituric acid
- TBST:
-
Tris-buffered saline tween
- TCA:
-
Trichloroacetic acid
- TNF-α:
-
Tumor necrosis factor-alpha
References
Akella A, Sharma P, Pandey R, Deshpande SB (2014) Characterization of oleic acid-induced acute respiratory distress syndrome model in rat. Indian J Exp Biol 52:712–719
Ballard-Croft C, Wang D, Sumpter LR, Zhou X, Zwischenberger JB (2012) Large-animal models of acute respiratory distress syndrome. Ann Thorac Surg 93:1331–1339
Bargi R, Asgharzadeh F, Beheshti F, Hosseini M, Sadeghnia HR, Khazaei M (2017) The effects of thymoquinone on hippocampal cytokine level, brain oxidative stress status and memory deficits induced by lipopolysaccharide in rats. Cytokine 96:173–184
Bhatia M, Moochhala S (2004) Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol 202:145–156
Borges AM, Ferrari RS, Thomaz LDGR, Ulbrich JM, Félix EA, Silvello D, Andrade CF (2019) Challenges and perspectives in porcine model of acute lung injury using oleic acid. Pulm Pharmacol Ther 59:101837
Chen S, Zheng S, Liu Z, Tang C, Zhao B, Du J, Jin H (2015) Endogeous sulfur dioxide protects against oleic acid-induced acute lung injury in association with inhibition of oxidative stress in rats. Lab Invest 95:142–156
Chen L, Li W, Qi D, Wang D (2018) Lycium barbarum polysaccharide protects against LPS-induced ARDS by inhibiting apoptosis, oxidative stress, and inflammation in pulmonary endothelial cells. Free Radic Res 52:480–490
Cui N, Liang Y, Wang J, Liu B, Wei B, Zhao Y (2021) Minocycline attenuates oxidative and inflammatory injury in a intestinal perforation induced septic lung injury model via down-regulating lncRNA MALAT1 expression. Int Immunopharmacol 100:108115
Eduviere A, Otomewo L (2022) Minocycline abrogates lung oxidative damage and haematological perturbations in mice exposed to hypoxia. J Drug Deliv Ther 12:82–90
Eisvand F, Imenshahidi M, Ghasemzadeh Rahbardar M, Tabatabaei Yazdi SA, Rameshrad M, Razavi BM, Hosseinzadeh H (2022) Cardioprotective effects of alpha-mangostin on doxorubicin-induced cardiotoxicity in rats. Phytother Res 36:506–524
Erdem A, Gedikli E, Yersal N, Karaismailoglu S, Muftuoglu S, Fadillioglu E, Tuncer M (2017) Protective role of erdosteine pretreatment on oleic acid–induced acute lung injury. J Surg Res 213:234–242
Garrido-Mesa N, Zarzuelo A, Gálvez J (2013) Minocycline: far beyond an antibiotic. Br J Pharmacol 169:337–352
Ghasemzadeh Rahbardar M, Razavi BM, Hosseinzadeh H (2020) Investigating the ameliorative effect of alpha-mangostin on development and existing pain in a rat model of neuropathic pain. Phytother Res 34:3211–3225
Gonçalves-de-Albuquerque CF, Silva AR, Burth P, Castro-Faria MV, Castro-Faria-Neto HC (2015) Acute respiratory distress syndrome: role of oleic acid-triggered lung injury and inflammation. Mediators Inflamm 2015:260465
Guo Q, Jin J, Yuan JX-J, Zeifman A, Shen B, Huang J (2011) VEGF, Bcl-2 and Bad regulated by angiopoietin-1 in oleic acid induced acute lung injury. Biochem Biophys Res Commun 413:630–636
Kalemci S, Dirican N, Cetin E, Sözen H, Uner A, Yaylali A, Aksun S, Karacam V, Ulger E, Sütcü R (2013) The efficacy of minocycline against methotrexate-induced pulmonary fibrosis in mice. Eur Rev Med Pharmacol Sci 17:3334–3340
Kelly K, Sutton T, Weathered N, Ray N, Caldwell E, Plotkin Z, Dagher P (2004) Minocycline inhibits apoptosis and inflammation in a rat model of ischemic renal injury. Am J Physiol Renal Physiol 287:F760–F766
Li W, Zhao X, Yu T-T, Hao W, Wang G-G (2021) Knockout of PKC θ gene attenuates oleic acid-induced acute lung injury via reduction of inflammation and oxidative stress. Iran J Basic Med Sci 24:986–991
Liu D, Luo G, Luo C, Wang T, Sun G, Hei Z (2015) Changes in the concentrations of mediators of inflammation and oxidative stress in exhaled breath condensate during liver transplantation and their relations with postoperative ARDS. Respir Care 60:679–688
Matute-Bello G, Frevert CW, Martin TR (2008) Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295:L379–L399
Mokra D, Kosutova P, Balentova S, Adamkov M, Mikolka P, Mokry J, Antosova M, Calkovska A (2016) Effects of budesonide on the lung functions, inflammation and apoptosis in a saline-lavage model of acute lung injury. J Physiol Pharmacol 67:919–932
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78
Ochsendorf F (2010) Minocycline in acne vulgaris. Am J Clin Dermatol 11:327–341
Pan L, Yao D-C, Yu Y-Z, Li S-J, Chen B-J, Hu G-H, Xi C, Wang Z-H, Wang H-Y, Li J-H (2016) Necrostatin-1 protects against oleic acid-induced acute respiratory distress syndrome in rats. Biochem Biophys Res Commun 478:1602–1608
Porter JC, Hall A (2009) Epithelial ICAM-1 and ICAM-2 regulate the egression of human T cells across the bronchial epithelium. FASEB J 23:492–502
Rahbardar MG, Eisvand F, Rameshrad M, Razavi BM, Hosseinzadeh H (2022) In vivo and in vitro protective effects of rosmarinic acid against doxorubicin-induced cardiotoxicity. Nutr Cancer 74:747–760
Seabrook TJ, Jiang L, Maier M, Lemere CA (2006) Minocycline affects microglia activation, Aβ deposition, and behavior in APP-tg mice. Glia 53:776–782
Shayan M, Mehri S, Razavi BM, Hosseinzadeh H (2022) Minocycline protects PC12 cells against cadmium-induced neurotoxicity by modulating apoptosis. Biol Trace Elem Res 201:1946–1954
Singh H, Kakkar AK, Chauhan P (2020) Repurposing minocycline for COVID-19 management: mechanisms, opportunities, and challenges. Expert Rev Anti Infect Ther 18:997–1003
Szeto GL, Brice AK, Yang H-C, Barber SA, Siliciano RF, Clements JE (2010) Minocycline attenuates HIV infection and reactivation by suppressing cellular activation in human CD4+ T cells. J Infect Dis 201:1132–1140
Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Acknowledgements
This work was supported by the Pharmaceutical Research Center and the Vice-Chancellor of Research, Mashhad University of Medical Sciences.
Funding
This research was supported by the Vice-Chancellor of Research, Mashhad University of Medical Sciences (No: 990628).
Author information
Authors and Affiliations
Contributions
HH and BMR were supervisors, designed the work, revised it critically for important intellectual content, and approved the version to be published. MGR did the experiment and wrote the manuscript, and KN helped in the pilot studies. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal studies were carried out in accordance with the norms of the Ethics Committee of Mashhad University of Medical Sciences (No: IR.MUMS.PHARMACY.REC.1399.066).
Consent for publication
All authors have agreed to the contents and approved the final version for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghasemzadeh Rahbardar, M., Razavi, B.M., Naraki, K. et al. Therapeutic effects of minocycline on oleic acid-induced acute respiratory distress syndrome (ARDS) in rats. Naunyn-Schmiedeberg's Arch Pharmacol 396, 3233–3242 (2023). https://doi.org/10.1007/s00210-023-02532-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02532-3