Abstract
Emerging evidence suggests that remdesivir might improve clinical outcome of high-risk outpatients with coronavirus disease 2019 (COVID-19). Our aim was to evaluate characteristics and outcomes of nonhospitalised adults diagnosed with COVID-19 and treated with early remdesivir therapy during the omicron wave. A single-centre prospective cohort study was performed among adult patients between February and June 2022, during the circulation of phylogenetic assignment of named global outbreak (PANGO) subvariants BA.2, BA.4, and BA.5 in Hungary. Patients were enrolled based on pre-defined criteria. Clinical characteristics (demography, comorbidities, vaccination status, imaging, treatment, and disease course) and outcomes (COVID-19 related hospitalisation, oxygen supplementation, intensive care support, and all-cause death) were assessed at 28 days post-treatment. A subgroup analysis of patients with and without active haematological malignancies was also carried out. Altogether, 127 patients were enrolled: 51.2% (65/127) were female with a median age of 59 (IQR: 22, range: 21‒92) years, and 48.8% (62/127) had active haematological malignancy. At 28 days post-treatment, 7.1% (9/127) of patients required COVID-19-related hospitalisation, 2.4% (3/127) required oxygen supplementation, 1.6% (2/127) required intensive care, and 0.8% (1/127) died due to a non-COVID-19-related secondary infection at the intensive care unit, all with haematological malignancies. Early remdesivir treatment might be a feasible strategy among high-risk outpatients with COVID-19 during the omicron wave.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Management strategies of patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) depend on the clinical context, patient-level risk factors, and treatment availability (IDSA 2022). Outpatients who are unvaccinated or partially immunised, as well as elderly, obese, or immunocompromised are at higher risk for severe or progressive disease (Gottlieb et al. 2022). Therefore, early initiation of therapy might be warranted in these subpopulations (Gottlieb et al. 2022).
Antiviral treatment options include monoclonal antibodies and direct antiviral agents (IDSA 2022). Monoclonal antibodies targeting spike protein of the SARS-CoV-2 have been shown to provide clinical benefit among certain clinical circumstances in treating coronavirus disease 2019 (COVID-19). However, the anticipated in vivo activity of different monoclonal antibodies greatly varies depending on the viral subvariant type, therefore limiting universal applicability (IDSA 2022). Currently, recommended antiviral drugs against SARS-CoV-2 are polymerase inhibitors remdesivir, molnupiravir, and the protease inhibitor combination nirmatrelvir/ritonavir (IDSA 2022). Remdesivir has already been licenced for in-hospital treatment of SARS-CoV-2-infected patients, but recent data support its use in early COVID-19 in the outpatient setting by lowering the overall risk of hospitalisation and disease progression (Lin et al. 2021; Panagopoulos et al. 2022).
Access to the different monoclonal antibodies and direct antiviral agents varies by country. In Hungary, particularly in the context of lacking oral antivirals during our study period, remdesivir became the single therapeutic option for high-risk outpatients. The aim of the present study was to evaluate clinical characteristics and outcomes of high-risk adults receiving early remdesivir treatment in the outpatient setting during the phylogenetic assignment of named global outbreak (PANGO) BA.2, BA.4, and BA.5 omicron subvariant predominance in Hungary (ECDC 2022).
Materials and methods
Study design and setting
A single-centre prospective cohort study was conducted among SARS-CoV-2-infected patients who were at high risk for disease progression and received early remdesivir therapy between February and June 2022. Our centre is a national-level referral institution of COVID-19 with a high-influx COVID-19 outpatient department during the pandemic (Supplementary File 1). The study design was in accordance with the Helsinki Declaration and national ethical standards. The study protocol was approved by the Institutional Review Board of South Pest Central Hospital, National Institute of Haematology and Infectious Diseases (IKEB-14/2020). Written informed consent was obtained from each included patient.
Patient eligibility and inclusion
Eligible high-risk patients were aged at least 18 years, had ongoing COVID-19 confirmed by SARS-CoV-2 nasopharyngeal sample positivity using real-time polymerase chain reaction (RT-PCR) and antigenic testing, and possessed ≥ 1 predefined patient-level risk factor for disease progression at inclusion. Predefined patient-level risk factors were essential hypertension, obesity (body mass index > 25 kg/m2), chronic cardiovascular disease, chronic cerebrovascular disease, chronic pulmonary disease, chronic renal disease, chronic liver disease, diabetes mellitus, immunocompromised state, and active oncological and haematological malignancies. All eligible patients were included consecutively at COVID-19 diagnosis if they consented to receive remdesivir for a minimum of 3 days and promptly started after diagnosis ascertainment. No a priori exclusion criteria were used. COVID-19 severity was given according to the World Health Organisation (WHO) criteria (WHO 2022). After inclusion completion, the cohort was stratified into two subgroups according to active haematological malignancy as a comorbidity.
Data collection
Patient data were collected anonymously through electronic medical records and clinical charts and recorded in a structural database. Data collected were 1) age and sex, 2) comorbidities, 3) COVID-19 vaccination status and previous SARS-CoV-2 infections, 4) disease course (onset of typical symptoms, disease severity), 5) nasopharyngeal and blood SARS-CoV-2 RT-PCR results, 6) results of chest computed tomography (CT) scans, 7) details of remdesivir treatment, and 8) clinical outcomes. Baseline variables were recorded at COVID-19 diagnosis.
Therapeutic strategies
Included patients received 200 mg remdesivir quaque die (QD) intravenously and diluted in 0.9% saline according to instructions of the manufacturer, and 100 mg remdesivir QD on the following days. The protocol suggested 3 days of standard treatment, starting on the day of COVID-19 diagnosis, but at the discretion of the attending physician, prolongation of treatment was permitted for a total of 5 days.
Outcomes and patient follow-up
The primary endpoint was the need for hospitalisation due to COVID-19. Secondary endpoints were all-cause death, need for oxygen supplementation, and need for intensive care unit admission. All outcome measures were assessed at + 28 days after the end of remdesivir treatment. Patient follow-up is detailed in Supplementary File 1.
Statistical analysis
Continuous variables are reported by median with interquartile range (IQR) and minimum–maximum ranges. The Shapiro–Wilk test was used for normality check. Categorical variables are reported as absolute numbers (n) with relative percentages (%). Student's t-test and chi-squared test were used for statistical comparison depending on variable distribution. Statistical significance was decided at a two-sided p-value of < 0.05 for all tests.
Results
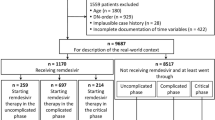
In total, 127 patients were enrolled during the study period (Table 1). Among them, 48.8% (n = 62) had an active haematological malignancy. These were non-Hodgkin lymphoma (n = 25; 40.3%), acute leukaemia (n = 16; 25.8%), chronic lymphocytic leukaemia (n = 10; 16.1%), myeloproliferative neoplasms (n = 4; 6.5%), Hodgkin’s lymphoma (n = 4; 6.5%), and myelodysplastic syndrome (n = 3; 4.8%); 51.2% (n = 65) of patients had a patient-level risk factor other than haematological malignancy. Median age at diagnosis was 59 (IQR: 22, range: 21–92) years, 51.2% (n = 65) of included patients were female in the total cohort. Age, sex, and comorbidities were equally distributed between subgroups, except for essential hypertension, and active oncological and systematic autoimmune diseases, which were statistically more frequent in the non-haematological subgroup. Presence of SARS-CoV-2 RNAemia was found at similar rates between subgroups. Upon chest CT examinations, eighteen patients (17.6%) in the total cohort had alterations typical for COVID-19. More patients were fully vaccinated in the non-haematological subgroup. The severity of symptoms was balanced. The median time from symptom onset to first dose of remdesivir was 3 (IQR: 3, range: 0–63) days, with no statistically significant difference between subgroups. From the total cohort, nine patients (7.1%) had to be admitted to the hospital, three (2.4%) required oxygen supplementation, two (1.6%) were transferred to intensive care unit, and one (0.8%) died within the study period. All negative outcomes occurred among patients with haematological malignancy. The 3-day therapy protocol was more frequently administered among non-haematological patients (38.6% vs. 12.9%, p < 0.01).
Discussion
Present study
This single-centre prospective cohort study evaluated clinical characteristics and outcomes of high-risk adult outpatients receiving early remdesivir therapy in the SARS-CoV-2 omicron variant era. We documented low rates of hospitalisation and all-cause death, and all negative outcomes occurring among patients with malignant haematological diseases.
Current literature
In the literature, multiple studies have assessed the therapeutic effect of an early 3-day remdesivir therapy among adult outpatients with a wide-spectrum of patient-level risk factors (Gottlieb et al. 2022; Panagopoulos et al. 2022). A randomised, double-blind, placebo-controlled study involving 562 nonhospitalised high-risk patients documented an 87% survival among patients receiving the 3-day remdesivir therapy compared to the placebo arm (Gottlieb et al. 2022). Also, a matched-pair retrospective study comparing COVID-19 related hospitalisation and acute respiratory failure among outpatients treated with a 3-day remdesivir to placebo reported a 75% lower rate of hospital admission and a 95% lower rate of acute respiratory failure among patients receiving remdesivir (Panagopoulos et al. 2022).
Clinical data concerning SARS-COV-2-infected patients with haematological malignancies during the omicron wave reports a higher risk for progression to severe disease and a reduced immune response post-vaccination, but decreased hospital admission and mortality rates were also documented (Blennow et al. 2022). Different comprehensive strategies such as remdesivir plus monoclonal antibodies or remdesivir plus convalescent plasma therapy proved to be successful for protection among patients with an active haematological malignancy (Cesaro et al. 2022). However, efficacy of monoclonal antibodies depends on the circulating variant, whereas accessibility of convalescent plasma therapy may be hindered in some low-income settings (IDSA 2022).
During the alpha and delta waves, monoclonal antibody therapies were generally available for the treatment of SARS-CoV-2-infected high-risk outpatients (IDSA 2022). In addition, in Hungary, favipiravir had been included in the national COVID-19 guideline based on an off-label approval by the National Institute of Pharmacy and Nutrition. Subsequent trials, however, did not confirm its positive effect on clinical outcomes in the early phase of COVID-19 (Szabo 2021; Chuah et al. 2022).
So far, two orally administered antivirals became available in some European countries with full or conditional approval by the European Medicine Agency. The cysteine protease inhibitor nirmatrelvir/ritonavir and the replication-inhibiting molnupiravir proved to be effective not only in in vitro studies but also in real-life settings during the PANGO BA.2 dominance (IDSA 2022; WHO 2022). These drugs are easy to administer and possess good bioavailability with generally acceptable side-effect profiles (IDSA 2022; WHO 2022). However, in countries with limited accessibility to oral antivirals against SARS-CoV-2, administration of remdesivir may be retained as an essential treatment strategy for COVID-19. Furthermore, among patients with haematological malignancies where viral persistence, longer viral shedding, and viraemia are a problematic tendency, a parenterally administered medication could be a rational option (Cesaro et al. 2022). This might be mirrored by the change in WHO recommendations where indication for remdesivir was expanded to the outpatient setting while also acknowledging its efficacy against SARS-CoV-2 PANGO variants BA.2, BA.4, and BA.5 (Gottlieb et al. 2022; WHO 2022).
Study limitations
The present study has some limitations. First, we did not identify SARS-CoV-2 variants of each patient. We considered the omicron variant of SARS-CoV-2 as dominant in Hungary based on surveillance samples to the European Centre for Disease Control and Prevention by national authorities (ECDC 2022). Second, a relatively small number of patients were involved in the study. In addition, as the study design is a simple observational study, no randomisation or placebo control was feasible in the outpatient setting for high-risk patients. In a pandemic situation with a high influx of patients per day, a throughout patient medical history taking was not always feasible; therefore, we might assume that some clinical data were biased during collection. The majority of patients in the cohort were at least partially vaccinated, which may partially account for lower hospitalisation and mortality rates. Lastly, we did not follow severe or critical cases from the study population over the pre-defined 28-day period; therefore, late outcomes could not be reported in our study.
Conclusion
In this single-centre prospective cohort study conducted during the SARS-CoV-2 omicron wave in the outpatient setting, outcomes of high-risk adults, including patients with haematological malignancies, treated with early remdesivir therapy were favourable, providing a clinically feasible strategy against COVID-19.
Data, material, and/or code availability
Anonymised data of patients are available from the corresponding author upon reasonable request.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- CT:
-
Computed tomography
- IQR:
-
Interquartile range
- PANGO:
-
Phylogenetic assignment of named global outbreak
- PCR:
-
Polymerase chain reaction
- QD:
-
Once daily [quaque die]
- RT-PCR:
-
Real-time polymerase chain reaction
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- WHO:
-
World Health Organisation
References
Blennow O, Salmanton-García J, Nowak P et al (2022) Outcome of infection with omicron SARS-CoV-2 variant in patients with hematological malignancies: an EPICOVIDEHA survey report. Am J Hematol 97:E312–E317. https://doi.org/10.1002/ajh.26626
Cesaro S, Ljungman P, Mikulska M et al (2022) Recommendations for the management of COVID-19 in patients with haematological malignancies or haematopoietic cell transplantation, from the 2021 European Conference on Infections in Leukaemia (ECIL 9). Leukemia 36:1467–1480. https://doi.org/10.1038/s41375-022-01578-1
Chuah CH, Chow TS, Hor CP et al (2022) Efficacy of early treatment with favipiravir on disease progression among high-risk patients with coronavirus disease 2019 (COVID-19): a randomized, open-label clinical trial. Clin Infect Dis 75:e432–e439. https://doi.org/10.1093/cid/ciab962
European Centre for Disease Prevention and Control (2022) SARS-CoV-2 variants of concern as of 22 September 2022. https://www.ecdc.europa.eu/en/covid-19/variants-concern. Accessed 22 October 2022
Gottlieb RL, Vaca CE, Paredes R et al (2022) Early remdesivir to prevent progression to severe Covid-19. N Engl J Med 386: 305–315. https://doi.org/10.1056/NEJMoa2116846
Infectious Diseases Society of America (2022) IDSA Guidelines on the Treatment and Management of Patients with COVID-19. https://www.covid19treatmentguidelines.nih.gov/. Accessed 22 October 2022
Lin HXJ, Cho S, Aravamudan VM et al (2021) Remdesivir in coronavirus disease 2019 (COVID-19) treatment: a review of evidence. Infection 49:401–410. https://doi.org/10.1007/s15010-020-01557-7
Panagopoulos P, Petrakis V, Trypsianis G, Papazoglou D (2022) Early 3-day course of remdesivir in vaccinated outpatients with SARS-CoV-2 infection. A success story. J Chemother Online ahead of print. https://doi.org/10.1080/1120009X.2022.2099693
Szabo BG, Lenart KS, Petrik B et al (2021) Favipiravir treatment does not influence disease progression among adult patients hospitalized with moderate-to-severe COVID-19: a prospective, sequential cohort study from Hungary. Geroscience 43:2205–2213. https://doi.org/10.1007/s11357-021-00452-9
World Health Organisation (2022) Living guidance for clinical management of COVID-19. https://apps.who.int/iris/bitstream/handle/10665/349321/WHO-2019-nCoV-clinical-2021.2-eng.pdf. Accessed 22 October 2022
Acknowledgements
The following authors from South Pest Central Hospital, National Institute of Hematology and Infectious Diseases collaborated with the preparation of the manuscript: Anita Ábrahám, Zsófia Balogh, Zsuzsanna Bányai, Emese Bányász, József Budai, Eszter Dános-Czél, Katalin Fried, Adrienn Hanuska, Mátyás Horváth, Nóra Jenőfi, János Kádár, Noémi Kiss-Dala, Katalin Klenjánszki, Botond Lakatos, Katalin Szidónia Lénárt, Csaba Lőrinczi, Éva Lívia Nagy, Krisztina Nemesi, Ákos Osvald, Edina Petrovicz, Alexandra Riczu, Zsuzsa Stelbaczky, Bálint Gergely Szabó, Judit Szanka, Andrea Szombati, Éva Terék, Szilvia Tóth, Zsuzsanna Várnai, Orsolya Wöller, and Anita Zempléni-Tóth. The authors would like to thank the healthcare workers of our center for their sacrifice during these times.
Funding
Open access funding provided by Semmelweis University.
Author information
Authors and Affiliations
Contributions
ZsG: data collection, data analysis, preparation of study protocol, and preparation of the manuscript; BGSz: management of patients, data analysis, preparation of study protocol, and preparation of the manuscript; AÁ: management of patients and review of the manuscript; ZsV: management of patients and review of the manuscript; NKD: management of patients and review of the manuscript; JSz: management of patients and review of the manuscript; JS: management of patients and review of the manuscript; VNI: preparation of study protocol and review of the manuscript; BL: management of patients, preparation of study protocol, data analysis, and preparation and review of the manuscript. Authors participated equally in patient management and manuscript revision. All authors have read and approved the final manuscript for publication. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
The study was in accordance with the Helsinki Declaration and national ethical standards. The institutional review board of South Pest Central Hospital, National Institute of Haematology and Infectious Diseases approved the study protocol. The National Institute of Pharmacy and Nutrition approved the off-label use of remdesivir detailed in the manuscript (https://www.ema.europa.eu/en/documents/product-information/veklury-epar-product-information_hu.pdf).
Consent to participate
Written informed consent was obtained from each patient.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the data in Table 1.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gáspár, Z., Szabó, B.G., Ábrahám, A. et al. Outcomes of high-risk adult outpatients treated with early remdesivir therapy during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) omicron era: experiences from the national centre of Hungary. Naunyn-Schmiedeberg's Arch Pharmacol 396, 1857–1862 (2023). https://doi.org/10.1007/s00210-023-02456-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02456-y