Abstract
This study aimed to demonstrate the potential benefits of donepezil (DPZ) and vitamin D (Vit D) in combination to counteract the neurodegenerative disorders induced by CuSO4 intake in experimental rats. Neurodegeneration (Alzheimer-like) was induced in twenty-four male Wistar albino rats by CuSO4 supplement to drinking water (10 mg/L) for 14 weeks. AD rats were divided into four groups: untreated AD group (Cu-AD) and three treated AD groups; orally treated for 4 weeks with either DPZ (10 mg/kg/day), Vit D (500 IU/kg/day), or DPZ + Vit D starting from the 10th week of CuSO4 intake. Another six rats were used as normal control (NC) group. The hippocampal tissue content of β-amyloid precursor protein cleaving enzyme 1 (BACE1), phosphorylated Tau (p-tau), clusterin (CLU), tumor necrosis factor-α (TNF-α), caspase-9 (CAS-9), Bax, and Bcl-2 and the cortical content of acetylcholine (Ach), acetylcholinesterase (AChE), total antioxidant capacity (TAC), and malondialdehyde (MDA) were measured. Cognitive function tests (Y-maze) and histopathology studies (hematoxylin and eosin and Congo red stains) and immunohistochemistry for neurofilament. Vit D supplementation alleviated CuSO4-induced memory deficits including significant reduction hippocampal BACE1, p-tau, CLU, CAS-9, Bax, and TNF-α and cortical AChE and MDA. Vit D remarkably increased cortical Ach, TAC, and hippocampal Bcl-2. It also improved neurobehavioral and histological abnormalities. The effects attained by Vit D treatment were better than those attained by DPZ. Furthermore, Vit D boosted the therapeutic potential of DPZ in almost all AD associated behavioral and pathological changes. Vit D is suggested as a potential therapy to retard neurodegeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia represents a serious global health crises of the twenty-first century with more than 50 million people worldwide suffer from dementia. This number is expected to triple to 152 million by 2050 as the world’s population ages. Alzheimer’s disease (AD) is the most frequent cause of dementia, accounting for 60–80% of all dementia cases (Porsteinsson et al. 2021). In AD, neuronal death and degeneration of neural connections in the cerebral cortex, as well as a significant loss of brain mass, take place. Within 5–10 years of commencement, AD is inevitably progressing and fatal. The actual factors that contribute to the development of AD are still unknown. Tau protein phosphorylation, changes in calcium metabolism, oxidative stress, neuroinflammation, improper energy metabolism, and abnormal formation and aggregation of amyloid beta (Aβ) are thought to be essential in the pathophysiology of AD (Anand et al. 2017).
Due to its complex etiology, no single drug or other intervention is likely to be effective in treating AD. The current treatment options for AD are only symptomatic and provoke a temporary relief in cases of mild to moderate AD. The only four medications currently approved by the Food and Drug Administration (FDA) for the management of AD are either acetylcholinesterase (AChE) inhibitors [donepezil (DPZ), galantamine, and rivastigmine] or N-methyl-D-aspartate (NMDA) receptor antagonists [memantine]. Of notice, there is no licensed medications that target the basic pathophysiology of AD to reduce or prevent the disease development (Rajasekhar and Govindaraju 2018). Although there are currently no successful treatments to slow its progression, many researchers have previously investigated several variables and biochemical biomarkers linked to AD in the hunt for effective cognitive impairment and dementia preventive measures. In addition, dietary and lifestyle changes, particularly vitamin supplementation, should be taken into consideration as it may help to slow the progression of AD (Jia et al. 2019).
Vitamin D (Vit D) is a steroid hormone that regulates calcium metabolism and bone health. Additionally, Vit D can cross the blood–brain barrier and its receptors and the enzymes associated with its activity are widely spread in the brain (Garcion et al. 2002). It therefore can affect brain activities; it was reported to improve cognitive performance, namely, attention, memory, orientation, and executive function (Lee et al. 2009; Przybelski and Binkley 2007), and decreases brain dysfunction in experimental models of diabetes (Alrefaie and Alhayani 2015), obesity (Hajiluian et al. 2018), multiple sclerosis (Smolders et al. 2008), and AD (Pogge 2010). In the brains of aged rats, Vit D reduced pro-inflammatory cytokines while increased anti-inflammatory cytokines (Briones and Darwish 2012). Moreover, Vit D has also been proven to promote Aβ plaque clearance responsible for cortical neurodegeneration, protect against apoptosis and oxidative stress, and upregulate the production of many neurotrophic factors that increase neuronal survival and function (Dursun et al. 2011; Fernandes de Abreu et al. 2009).
Copper is one of the heavy metals that have a strong binding affinity for amyloid precursor protein (APP) and Aβ, and it is thought that the presence of copper in the brain may aid in the development and aggregation of Aβ. Additionally, the chronic copper intake increased Aβ pathology and hindered cognitive function in animal models of AD (Kitazawa et al. 2009).
The present study aimed to (i) investigate the neuroprotective effects of Vit D on AD-like neurodegeneration induced by CuSO4, (ii) compare the effect of Vit D and DPZ that is routinely used to treat AD, (iii) demonstrate the therapeutic potential of drug combination strategy using Vit D and DPZ combination on multiple brain markers using neurodegeneration rat model induced by CuSO4 intake, and (iv) to demonstrate any correlation between AD biomarkers and the AD-related histopathological findings in brain hippocampus.
Materials and methods
Animals
Male Wister rats weighing about 150 ± 10 g were provided by the animal unit at the Faculty of Veterinary Medicine, Zagazig University, Egypt. The rats were housed in wire-floored cages and located under standard environmental conditions (temperature, 22 ± 2 °C; humidity, 50 ± 5%; night/day cycle, 12 h) with free access to standard pellet diet and tap water ad libitum. The experimental design and protocols were approved by the Institutional Animal Care and Use Committee of Zagazig University (ZU-IACUC/3/F/43/2019).
Drug administration and experimental design
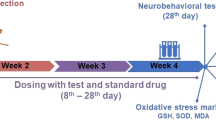
After a 2-week acclimatization period, thirty rats were randomly divided into five groups (n = 6 rats each). The experimental design is graphically represented in Fig. 1. Rats in normal control (NC) group received normal drinking water for 14 weeks. The other four groups were designated as the experimental groups in which rats were allowed to free access of drinking water containing 10 mg/L CuSO4 (Sigma Aldrich, St. Louis, MO, USA) for 14 weeks to develop AD-like neurodegeneration. The used dose of CuSO4 in drinking water and the 14-week duration were decided from a pilot study that revealed impaired cognitive function and β-amyloid deposits in hippocampus tissue (data not shown). Rats in these groups received vehicle or treatment for 4 weeks by oral gavage starting on the 10th week of CuSO4 administration as follows:
-
Cu-AD group: rats received drug vehicle (0.5 mL/rat/day).
-
DPZ group: rats received DPZ (Pfizer Egypt, Cairo, A.R.E; 10 mg/kg/day) (Kwon et al. 2014).
-
Vit D group: rats received Vit D (Medical Union Pharmaceuticals, Egypt; 500 IU/kg/day) (Hajiluian et al. 2017, Salum et al. 2012).
-
DPZ + Vit D group: rats received both DPZ and Vit D as previously described.
Y-maze behavior test (see below) was conducted 2 days prior to scarification. Rats were quickly anesthetized with 3% isoflurane and were sacrificed by rapid decapitation at the end of 14 weeks. The brains were immediately removed and were rinsed in ice-cold saline solution. The cerebral cortex and hippocampus were carefully dissected, and part was frozen in liquid nitrogen, and stored at − 80 °C for subsequent biochemical assessment. The other part of hippocampus was kept in 10% buffered formalin for histopathological and immunopathological studies.
Behavioral study
Y-maze test
The Y-maze test assessed spatial working memory ability. In the Y-maze, there were three identical arms at 120°: A, B, and C (length: 50, width: 16, height: 32 cm). Each rat was first placed in the center of the maze and given 5 min to explore freely. A successful spontaneous reaction alternation is considered when the rat’s head and limbs completely enter the arm with three different arms sequentially entered. In the experiment, after each test, the device is sprayed with 10% alcohol, scrubbed evenly with an absorbent cloth, and finally dried with tissue paper to ensure no attraction to animals to any arm. The spontaneous alternation rate (%) = [n/(N − 2) × 100] where “N” represented the total number of arm entries and “n” represented the successful entry sequence (Kang et al. 2016).
Biochemical study
Ach and AChE measurement
The content of Ach and AChE in brain cortex was measured using the commercially available rat-specific ELISA kits purchased from Elabscience, TX, USA; Catalog No: E-EL-0081 and E-EL-R0355, respectively.
Bax, Bcl-2, CAS-9, and TNF-α measurement
The brain hippocampal content of Bax, Bcl-2, CAS-9, and TNF-α was measured using ELISA kits purchased from Elabscience, TX, USA (Catalog No: E-EL-R0098, E-EL-R0096, E-EL-R0163, and E-EL-R0019, respectively).
BACE1, CLU, and p-tau measurement
The brain hippocampal content of BACE1, CLU, and p-tau was measured using commercially available ELISA kits. Rat BACE1 (Catalog No: LS-F15104), rat CLU (Catalog No: ELR-Clusterin), and rat p-tau (Catalog No: E-EL-R1090) kits were purchased from Lifespan Biosciences, Inc. (WA, USA), Raybiotech (GA, USA), and Elabscience (TX, USA), respectively.
TAC and MDA measurement
The total antioxidant capacity (TAC) and lipid peroxidation, expressed as malondialdehyde (MDA), were measured in cortex of brain tissue by using colorimetric method. Kits were purchased from Biodiagnostic Co., Giza, Egypt (Catalog No: TA 2512 and MD 2529, respectively).
Histopathology studies
Hippocampus Sections (5 µm thick) were stained with hematoxylin and eosin using standard histological procedures (H and E) (Serrano-Pozo et al. 2011). β-Amyloid deposits in hippocampus tissue were observed using Congo red staining (Khurana et al. 2001). A light microscope was used to examine the slides. ImageJ software was used to count the number of apoptotic neurons and amyloid deposits in hippocampus tissue.
Immunohistochemistry
For immunohistochemical staining of neurofilaments in hippocampal tissue, the mouse monoclonal light neurofilament primary antibody (Abcam, ab7255) was incubated overnight at 4 °C in a humid chamber with 5-µm hippocampus sections. After washing with phosphate buffer saline (PBS), a biotin-labeled goat anti-mouse IgG (Abcam, ab6788) was used as a secondary antibody. Microscopically, the sections were examined for specific staining (Olympus CX40; Olympus, Tokyo, Japan). The integrated optical density (IOD) value of the collected pictures was measured in 18 random visual fields from each group using ImageJ software.
Statistical analysis
GraphPad Prism version 5.0 was used for statistical analyses (GraphPad Software, San Diego, USA). Analysis of variance (ANOVA) was used to compare data, followed by Tukey’s t-test taking p < 0.05 as statistically significant. All results were graphically displayed as mean ± SD.
Results
Vit D improved spatial learning and memory ability in CuSO4-induced AD rats
When compared to the NC group, the consumption of CuSO4 in drinking water for 14 weeks significantly impaired locomotor activity (arm entry) and spontaneous alteration rate (p < 0.001). Vit D, DPZ, or their combination significantly reversed these CuSO4-induced deficits as shown in Table 1.
Vit D improved cholinergic activity in CuSO4-induced AD rats
CuSO4 exposure markedly increased cortical AChE while decreased cortical Ach content as compared to NC group (p < 0.001). In comparison to the Cu-AD group, treatment with Vit D, DPZ, or their combination considerably suppressed cortical AChE content and hence increased cortical Ach content (p < 0.001). Of notice, Vit D improved cholinergic activity better than DPZ (p < 0.001) and was even better than that attained by DPZ and Vit D combination (p < 0.001) as shown in Fig. 2.
Cholinergic activity (A) cortical AChE content and (B) cortical Ach content were measured in rats given CuSO4 (Cu-AD group) and treated for 4 weeks with DPZ, Vit D, or a combination of the two. Results are expressed as mean ± SD, n = 6. *p < 0.001 and Фp < 0.05 compared to NC group, #p < 0.001 compared to Cu-AD group, &p < 0.001 and ¥p < 0.05 compared to DPZ group, $p < 0.001 compared to Vit D group
Vit D constrained AD specific markers in CuSO4-induced AD rats
Rat from Cu-AD group had significantly higher hippocampal content of BACE1, CLU, and p-tau than NC rats (p < 0.001). Treatments with DPZ, Vit D, or their combination significantly reduced BACE1, CLU, and p-tau levels when compared to the untreated Cu rats (p < 0.001). Vit D outperformed DPZ and DPZ + Vit D (p < 0.001) in improving the biomarkers for AD (Fig. 3).
The brain hippocampal content of (A) BACE1, (B) CLU, and (C) p-tau in CuSO4-induced AD rats treated for 4 weeks with DPZ, Vit D, or their combination. Results are expressed as mean ± SD, n = 6. *p < 0.001 and Фp < 0.05 compared to NC group, #p < 0.001 compared to Cu-AD group, &p < 0.001 compared to DPZ group, $p < 0.001 compared to Vit D group
Vit D retarded apoptosis in CuSO4-induced AD rats
Rats from the Cu-AD group had more Bax, Bax/Bcl2 ration, and CAS-9 in their hippocampus than those from the NC group (p < 0.001). DPZ, Vit D, or their combination significantly reduced these apoptotic proteins in hippocampus (p < 0.001). Inversely, Cu-AD rats had lower hippocampal Bcl-2 content than NC rats (p < 0.001). Individual drugs and their combinations increased hippocampal Bcl-2 content when compared to the untreated Cu-AD group (p < 0.05 for DPZ and p < 0.001 for Vit D and combination therapy). Vit D was superior to both DPZ and combination therapy in increasing the anti-apoptotic Bcl-2 (p < 0.001 and p < 0.05, respectively) and decreasing apoptotic Bax (p < 0.001 compared to DPZ) and CAS-9 (p < 0.001 and p < 0.05, respectively) as shown in Fig. 4.
Biomarkers of hippocampal apoptosis (A) Bax, (B) Bcl-2, (C) Bax/Bcl-2 ratio, and (D) CAS-9 in rats received CuSO4 and treated with DPZ, Vit D, or their combination for 4 weeks. Results are expressed as mean ± SD, n = 6. *p < 0.001 and Фp < 0.05 compared to NC group, #p < 0.001 and @p < 0.05 compared to Cu-AD group, &p < 0.001 and ¥p < 0.05 compared to DPZ group, $p < 0.001 and €p < 0.05 compared to Vit D group
Vit D suppressed inflammation and oxidative stress in CuSO4-induced AD rats
Rats from Cu-AD group had significantly higher hippocampal TNF-α and cortical MDA content than NC rats (p < 0.001). However, cortical TAC was lower in Cu-AD rats than NC rats (p < 0.001). When compared to untreated Cu-AD rats, treatment with DPZ, Vit D, or their combination significantly reduced hippocampal TNF-α and cortical MDA content, while increased cortical TAC content (p < 0.001). Vit D produced remarkable results reducing hippocampal TNF-α and cortical MDA content when compared to DPZ and DPZ + Vit D treatments (p < 0.001 for TNF-α and p < 0.05 for MDA) as shown in Table 2. Cortical MDA was correlated with AChE (r = 0.87), Ach (r = − 0.87), BACE1 (r = 0.90), CLU (r = 0.85), and p-tau (r = 0.89). On the other hand, cortical TAC was correlated with AChE (r = − 0.87), Ach (0.85), BACE-1 (− 0.88), CLU (− 0.88), and p-tau (r = − 0.86).
Hematoxylin and eosin (H&E) staining
The actual hippocampus is divided into four sections known as CA1–4. The CA1 region of the hippocampus outflow is the first region to provide a significant yield route to the entorhinal subiculum and entorhinal cortex. The molecular, pyramidal, and polymorphic layers of the CA1 neurons in the hippocampus were stained with H&E. In NC rats, the pyramidal neuron cells were densely packed with large, spherical, brilliant vesicular nuclei with extensive cytoplasmic processes concentrated near the molecular layer. Supporting neuroglial cells, unmyelinated nerve axons, and dendrites abound in the neuropil were noticed. The neuroglial cells’ nuclei were dark-stained. Some pyramidal cells in the hippocampus CA1 area shrank dramatically in the Cu-AD group. With a significant decline in the pyramidal neuron cell population, their nuclei were relatively darkly pigmented and/or scattered. The nuclei of certain pyramidal nerve cells were bright. Gliosis was also represented by a large number of dark neuroglial cells near the nerve cell bodies. DPZ treatment prevented the loss of neuronal cell mass in the CA1 region of the hippocampus. The density of pyramidal nerve cells in the CA1 area increased significantly, with bright rounded vesicular nuclei with noticeable nucleoli and a decrease in the density of neuroglial cells. In the neuropil, several vacuoles were visible. Rats administered Vit D had a notable increase in neuronal cell mass, which had brilliant vesicular nuclei with noticeable nucleoli. When compared to Cu-AD rats, the neuropil presented a picture that was close to normal histological architecture with a significant decrease in neuroglial cells. The combination of DPZ and Vit D had a positive effect on nerve cell density, but it was less than Vit D alone as shown in Fig. 5.
H and E stained hippocampus sections of different experimental groups. The CA1 region of the hippocampus (the NC group (a) showing pyramidal layer (P), molecular layer (M), and polymorphic (PO) with bright vesicular nerve nuclei (n) and darkly stained nuclei of the neuroglial supporting cells (N) along with blood vessels (B) jamming the whole neuropil Cu-AD rats (b) showed shrunken nerve cells with darkly stained nuclei (*) with increased number of neuroglial cells (N). Some neurons were noticed with bright nuclei (n). DPZ-treated group (c) showed increased neuronal cell quantity (n) with their supporting neuroglial cells (N). Some neurons were also seen with darkly stained nuclei (*). Vit D-treated rats (d) showed a marked improvement in the nerve cell population (n) with less supporting glial cells (N). Fewer neurons were seen with darkly stained (*). The combination of both DPZ with Vit D (e) exhibited improvement of the histological structure of the neuropil. H and E, scale bar 50 µm, 400 × . The percent of apoptotic neurons (f) in different experimental group. Results are expressed as mean ± SD, n = 18. *p < 0.001 compared to NC group, #p < 0.001 compared to Cu-AD group, &p < 0.001 compared to DPZ group, €p < 0.05 compared to Vit D group
The deposition of amyloid plaques in different experimental group
The buildup of amyloid plaques in the hippocampus tissue of the CuSO4-ingested rat group was assessed using Congo red stain. In the NC group, the hippocampus CA1 nerve cells were found to be structured, with defined cell borders and brilliant vesicular nuclei. There were also visible nucleoli. Control animals showed no buildup of Congo red stain. However, the CA1 neurons Cu-AD rats had an asymmetrical architecture and appeared shrunken and bounded by Congo red staining which were prominent in the polymorphic and molecular layers. In comparison to the hippocampus of the Cu-AD group, the hippocampus of the DPZ, Vit D, and DPZ plus Vit D treated rats showed less amyloid plaques. When comparing the Vit D-treated rats to the other groups, the maximum protection was achieved by Vit D treatment as shown in Fig. 6. Of notice, amyloid plaque area was positively correlated with p-tau (r = 0.89), BAX (r = 0.84), and CAS 9 (r = 0.88) while negatively correlated with Bcl-2 (r = − 0.92).
Congo red paraffin-stained hippocampus sections from (a) NC rats; (b) Cu-AD rats; (c) DPZ-treated rats; (d) Vit D-treated rats; and (e) DPZ + Vit D-treated rats. (f) Amyloid deposits revealed by Congo red stain were measured using ImageJ software. Arrow denotes a positive reaction. Congo red stain, scale bar 50 µm, 400 × . Results are expressed as mean ± SD, n = 18. *p < 0.001 compared to NC group, #p < 0.001 compared to Cu-AD group, &p < 0.001 compared to DPZ group, $p < 0.001 compared to Vit D group
The expression of neurofilaments
The CA1 area of the hippocampus of the NC group was stained immunohistochemically and revealed normal pyramidal cells with brilliant vesicular nuclei. The neuropil exhibited no abnormal changes. Ingestion of CuSO4 in drinking water resulted in a significant decrease in the pyramidal cell population and a robust positive immune reaction to neurofilament (p < 0.001) compared to NC rats, indicating Tau hyperphosphorylation. When compared to Cu-AD rats, DPZ caused a notable increase in pyramidal cell density and decreased Tau protein (p < 0.001) expression. The nuclei of the majority of nerve cells are brilliant. When compared to Cu-AD rats, treatment with Vit D resulted in a remarkable increase in pyramidal cell mass and a significant decrease in immunoreaction to neurofilament (p < 0.001). In comparison to Cu-AD rats, the combination of Vit D and DPZ increased pyramidal cell survival and decreased neurofilament production. Figure 7 shows the total average intensity of immuno-stained areas. Of notice, the area of neurofilament immunostaining was positively correlated with p-tau (r = 0.87), BAX (r = 0.84), and CAS 9 (r = 0.89) while negatively correlated with Bcl-2 (r = − 0.71).
The expression of neurofilaments in the CA1 region of the hippocampus from (a) NC rats, (b) Cu-AD rats, (c) DPZ-treated rats, (d) Vit D-treated rats, and (e) DPZ + Vit D-treated rats. (f) The expression of neurofilaments measured using ImageJ software (results were expressed as mean ± SD, n = 18, *p < 0.001 compared to NC group, #p < 0.001 compared to Cu-AD group, &p < 0.001 compared to DPZ group, $p < 0.001 compared to Vit D group). Asterisk (*) denotes positive immunological reaction (neurofibrillary tangles immunostaining, scale bar 30 µm, 100 ×)
Discussion
Nutritional elements are vital in maintaining good health, and clinical data shows a link between nutritional deficiency and cognitive impairment (Dominguez and Barbagallo 2018). Vit D appears to play a role in brain growth and function, according to a substantial number of studies (Annweiler et al. 2015; Brondum-Jacobsen et al. 2013; Buell et al. 2010). Vit D possesses immune-modulating functions which can increase the expression of synapse structure proteins, neurotrophic factors, and depleted neurotransmitters in certain neurodegenerative disorders. Its deficiency may exacerbate inflammation and oxidative damage to neurons, leading to cognitive decline (Nourhashemi et al. 2018). In this investigation, we observed that the administration of Vit D effectively improved cognition, namely, spatial cognition ability tested by Y-maze in CuSO4-induced brain damage (neurodegeneration) in rats. It also retarded the progression of different AD-related changes which were remarkable compared to that of the standard drug DPZ.
Vit D regulates the expression of several neurotransmitters such as Ach and dopamine, as well as the enzyme involved in the rate-limiting step of catecholamine synthesis (Baksi and Hughes 1982; Puchacz et al. 1996; Sonnenberg et al. 1986). The central cholinergic system regulates neurocognitive functions via its neurotransmitter Ach and is implicated in a variety of neurodegenerative diseases, including AD. Cholinergic innervation is abundant in the cortex and hippocampal regions, and any neuronal loss in these brain regions can lead in altered cognitive status (Arendt et al. 1995; Bobinski et al. 1998). Cholinergic degeneration has been linked to the progression of clinical manifestations of AD in humans and experimental animal models, and AChE inhibitors have been widely used clinically to combat the symptoms cognitive impairment (Droguerre et al. 2020; Jiang et al. 2019; Sonkusare et al. 2005). Our results showed increased AChE along with decreased Ach in the brain cortex of Cu-AD group, whereas Vit D, DPZ, or their combination significantly decreased AChE which in turn increased Ach when compared to untreated Cu-AD group. Consistent with previous reports, our findings show that vitamin D supplementation improved cholinergic and memory functions (Kulkarni et al. 2022; Yamini et al. 2018).
These changes in cholinergic activity were highly interconnected with other AD-related pathogenesis such as the accumulation of Aβ plaques in brain hippocampal tissue (Congo red stain). The latter results from the abnormal cleavage of APP with BACE1 and is therefore considered as a hallmark in AD development (Anand et al. 2017). Increased AChE was found to stimulate the formation of Aβ plaques and enhance its incorporation into the growing Aβ-fibrils impairing synaptic conduction (De Ferrari et al. 2001). Moreover, the accumulated Aβ can directly interact with the high-affinity choline transporter diminishing Ach release (Bales et al. 2006). The deposition of Aβ peptides is markedly correlated with normal and pathological aging (Peng et al. 2021). In elderly patients suffering from AD, Vit D supplementation (800 IU/day) orally for 12 months improved cognitive function and decreased Aβ-related plasma biomarkers including Aβ42, APP, and BACE1 (Jia et al. 2019). In this context, our results showed a significant increase in hippocampal BACE1, CLU, and p-tau protein levels in untreated Cu-AD rats, whereas treatment with DPZ, Vit D, and their combination resulted in significant decrements in the levels of all aforementioned AD-related biomarker. These findings are in agreement with previous observations (Cheng et al. 2017, Grimm et al. 2014, Grimm et al. 2017, Lin et al. 2020).
The brain, as a metabolically active organs, is extremely prone to free radical-induced oxidative tissue damage due to limited availability of endogenous defense systems (Butterfield 2006). Therefore, the increased neuronal oxidative stress caused by excess Cu2+ exposure induced neuronal damage by affecting major cellular biomolecules leading to the development and progression of neurodegenerative disorders such as AD (Kumar et al. 2016). Our findings indicate that the oxidative profile in untreated Cu-AD rats was altered (increased cortical MDA and decreased cortical TAC) which were in strongly significant correlations with the changes in cholinergic activity, BACE1 and the accumulation of Aβ plaques in brain hippocampal tissue. The increased MDA in the brain cortex may be a result of accumulated Aβ oligomers which become a source of reactive oxygen species (ROS) initiating lipid peroxidation (Butterfield et al. 2002, 2001). Furthermore, the current study confirmed the role of Vit D either individually or in combination with DPZ in alleviating such oxidative stress in Cu-AD group (Vit D decreased cortical MDA while increased cortical TAC). Vit D also reduced the inflammatory damage induced by CuSO4 in the brain hippocampus, which was linked to neuronal injury reversal and enhanced cell survival. Also, the treatment of Cu-AD rats with DPZ, Vit D, and DPZ + Vit D significantly decreased hippocampal TNF-α content compared to the untreated Cu-AD rats. Comparable results were reported in rats with sporadic AD induced by intracerebrovascular-STZ. In these rats, the prophylactic vitamin D3 supplementation (42 IU) ameliorated behavioral changes, oxidative stress, and neuroinflammation improving cholinergic system and retarded brain degeneration (Yamini et al. 2018). Of notice, this increased oxidative stress can stimulate AChE (Melo et al. 2003) which further worsen cholinergic activity and supporting the beneficial use of antioxidants as Vit D on cholinergic activity (Medhat et al. 2020).
Neuronal apoptosis plays a crucial role in cognitive dysfunction. The ratio between Bcl-2/Bax, an important predictor of cell survival, can indicate the cell response to apoptotic stimuli. Low Bax/Bcl-2 ratio indicates the cellular ability to resist apoptotic stimuli, whereas a high ratio causes cell death (Jang et al. 2014). The present findings showed decreased Bcl-2 along with increased CAS-9 and Bax in the brain hippocampus of our untreated Cu-AD rats. The structural changes in the hippocampus of our Cu-AD rats also revealed increased apoptotic neurons. The interplay between the generation of ROS and apoptosis can in part explain the increased apoptosis in AD rats (Arowoogun et al. 2021, Turunc et al. 2014). Additionally, this apoptosis may be a result of cytoskeleton disruption due to the abnormal accumulation of phosphorylated Tau which can facilitate apoptosis and accelerate neurodegeneration (de la Monte 2012). In confirmation, the expression of neurofilaments, component of the cytoskeleton that constitutes neurofibrillary tangles, is increased in the hippocampal tissue of our Cu-AD rats. These neurofilaments are located in the same cellular compartment as microtubules and Tau (Rajasekhar and Govindaraju 2018).
Alternatively, the treatment with DPZ, Vit D, and DPZ + Vit D significantly reduced the Bax/Bcl-2 ratio and cleaved CAS-9. These treatments also reduced apoptotic neurons and neurofilament expression in the hippocampal tissue. Similarly, Yan and colleagues reported that calcitriol (2 μg/kg, ip for 4 weeks) can ameliorate cognitive decline in mice with systemic lupus erythematosus by hindering hippocampal neuron apoptosis (lowering hippocampal cleaved caspase-3 and increasing Bcl-2) (Yan et al. 2019). Vitamin D was also reported to downregulate the gene expression of caspase-3 and Bax and to upregulate the gene expression of Bcl-2 and hence alleviated cognitive dysfunction in AD mouse model (Bao et al. 2020). Of notice, the expression of neurofilaments in the hippocampal tissue is linked to p-tau and apoptotic markers.
The addition of Vit D to DPZ improved the therapeutic potential of DPZ, although it was not as good as Vit D alone. The effects of Vit D were even better than those attained by DPZ alone owing to multiple effects of Vit D on most pathological changes associated with CuSO4 administration and the AD-like neurodegenerative changes. Further investigation may be required to settle the nature of this combined therapy.
Conclusion
Our results indicated that Vit D supplementation was beneficial in alleviating CuSO4-induced memory deficits including neurobehavioral, biochemical (cholinergic activity, Aβ formation cascade, oxidative stress, apoptosis), and histological abnormalities. Although vitamin D alone revealed better activities than the reference AChE inhibitor, DPZ, adding Vit D to DPZ improved the therapeutic potential of DPZ in almost all AD-associated behavioral and pathological changes.
Data availability
Data are available to the corresponding author upon reasonable request.
Ethical approval
All experiments and animal procedures received ethical approval from the Institutional Animal Care and Use Committee of Zagazig University (ZU-IACUC/3/F/43/2019).
Change history
29 August 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s00210-023-02690-4
References
Alrefaie Z, Ae A (2015) Vitamin D3 improves decline in cognitive function and cholinergic transmission in prefrontal cortex of streptozotocin-induced diabetic rats. Behav Brain Res 287:156–162. https://doi.org/10.1016/j.bbr.2015.03.050
Anand A, Patience AA, Sharma N, Khurana N (2017) The present and future of pharmacotherapy of Alzheimer’s disease: a comprehensive review. Eur J Pharmacol 815:364–375. https://doi.org/10.1016/j.ejphar.2017.09.043
Annweiler C, Bartha R, Karras SN, Gautier J, Roche F, Beauchet O (2015) Vitamin D and white matter abnormalities in older adults: a quantitative volumetric analysis of brain MRI. Exp Gerontol 63:41–47. https://doi.org/10.1016/j.exger.2015.01.049
Arendt T, Bruckner MK, Bigl V, Marcova L (1995) Dendritic reorganisation in the basal forebrain under degenerative conditions and its defects in Alzheimer’s disease. III. The basal forebrain compared with other subcortical areas. J Comp Neurol 351:223–246. https://doi.org/10.1002/cne.903510204
Arowoogun J, Akanni OO, Adefisan AO, Owumi SE, Tijani AS, Adaramoye OA (2021) Rutin ameliorates copper sulfate-induced brain damage via antioxidative and anti-inflammatory activities in rats. J Biochem Mol Toxicol 35:e22623. https://doi.org/10.1002/jbt.22623
Baksi SN, Hughes MJ (1982) Chronic vitamin D deficiency in the weanling rat alters catecholamine metabolism in the cortex. Brain Res 242:387–390. https://doi.org/10.1016/0006-8993(82)90331-6
Bales KR, Tzavara ET, Wu S, Wade MR, Bymaster FP, Paul SM, Nomikos GG (2006) Cholinergic dysfunction in a mouse model of Alzheimer disease is reversed by an anti-A beta antibody. J Clin Invest 116:825–832. https://doi.org/10.1172/JCI27120
Bao Z, Wang X, Li Y, Feng F (2020) Vitamin D alleviates cognitive dysfunction by activating the VDR/ERK1/2 signaling pathway in an Alzheimer’s disease mouse model. NeuroImmunoModulation 27:178–185. https://doi.org/10.1159/000510400
Bobinski M, de Leon MJ, Tarnawski M, Wegiel J, Reisberg B, Miller DC, Wisniewski HM (1998) Neuronal and volume loss in CA1 of the hippocampal formation uniquely predicts duration and severity of Alzheimer disease. Brain Res 805:267–269. https://doi.org/10.1016/s0006-8993(98)00759-8
Briones TL, Darwish H (2012) Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. J Neuroinflammation 9:244. https://doi.org/10.1186/1742-2094-9-244
Brondum-Jacobsen P, Nordestgaard BG, Schnohr P, Benn M (2013) 25-Hydroxyvitamin D and symptomatic ischemic stroke: an original study and meta-analysis. Ann Neurol 73:38–47. https://doi.org/10.1002/ana.23738
Buell JS et al (2010) 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology 74:18–26. https://doi.org/10.1212/WNL.0b013e3181beecb7
Butterfield DA (2006) Oxidative stress in neurodegenerative disorders. Antioxid Redox Signal 8:1971–1973. https://doi.org/10.1089/ars.2006.8.1971
Butterfield DA, Drake J, Pocernich C, Castegna A (2001) Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol Med 7:548–554. https://doi.org/10.1016/s1471-4914(01)02173-6
Butterfield DA, Castegna A, Lauderback CM, Drake J (2002) Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol Aging 23:655–664. https://doi.org/10.1016/s0197-4580(01)00340-2
Cheng J, Rui Y, Qin L, Xu J, Han S, Yuan L, Yin X, Wan Z (2017) Vitamin D combined with resveratrol prevents cognitive decline in SAMP8 mice. Curr Alzheimer Res 14:820–833. https://doi.org/10.2174/1567205014666170207093455
De Ferrari GV, Canales MA, Shin I, Weiner LM, Silman I, Inestrosa NC (2001) A structural motif of acetylcholinesterase that promotes amyloid beta-peptide fibril formation. Biochemistry 40:10447–10457. https://doi.org/10.1021/bi0101392
de la Monte SM (2012) Contributions of brain insulin resistance and deficiency in amyloid-related neurodegeneration in Alzheimer’s disease. Drugs 72:49–66. https://doi.org/10.2165/11597760-000000000-00000
Dominguez LJ, Barbagallo M (2018) Nutritional prevention of cognitive decline and dementia. Acta Biomed 89:276–290. https://doi.org/10.23750/abm.v89i2.7401
Droguerre M et al (2020) Efficacy of THN201, a combination of donepezil and mefloquine, to reverse neurocognitive deficits in Alzheimer’s disease. Front Neurosci 14:563. https://doi.org/10.3389/fnins.2020.00563
Dursun E, Gezen-Ak D, Yilmazer S (2011) A novel perspective for Alzheimer’s disease: vitamin D receptor suppression by amyloid-β and preventing the amyloid-β induced alterations by vitamin D in cortical neurons. J Alzheimers Dis 23:207–219. https://doi.org/10.3233/JAD-2010-101377
Fernandes de Abreu DA, Eyles D, Féron F (2009) Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 34:S265–S277. https://doi.org/10.1016/j.psyneuen.2009.05.023
Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D (2002) New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab 13:100–105. https://doi.org/10.1016/s1043-2760(01)00547-1
Grimm MO et al (2014) Impact of vitamin D on amyloid precursor protein processing and amyloid-beta peptide degradation in Alzheimer’s disease. Neurodegener Dis 13:75–81. https://doi.org/10.1159/000355462
Grimm MOW, et al. (2017) Vitamin D and its analogues decrease amyloid-beta (Abeta) formation and increase Abeta-degradation. Int J Mol Sci 18. https://doi.org/10.3390/ijms18122764
Hajiluian G, Nameni G, Shahabi P, Mesgari-Abbasi M, Sadigh-Eteghad S, Farhangi MA (2017) Vitamin D administration, cognitive function, BBB permeability and neuroinflammatory factors in high-fat diet-induced obese rats. Int J Obes (lond) 41:639–644. https://doi.org/10.1038/ijo.2017.10
Hajiluian G, Abbasalizad Farhangi M, Nameni G, Shahabi P, Megari-Abbasi M (2018) Oxidative stress-induced cognitive impairment in obesity can be reversed by vitamin D administration in rats. Nutr Neurosci 21:744–752. https://doi.org/10.1080/1028415X.2017.1348436
Jang TY, Park CS, Kim KS, Heo MJ, Kim YH (2014) Benzaldehyde suppresses murine allergic asthma and rhinitis. Int Immunopharmacol 22:444–450. https://doi.org/10.1016/j.intimp.2014.07.029
Jia J, Hu J, Huo X, Miao R, Zhang Y, Ma F (2019) Effects of vitamin D supplementation on cognitive function and blood Abeta-related biomarkers in older adults with Alzheimer’s disease: a randomised, double-blind, placebo-controlled trial. J Neurol Neurosurg Psychiatry 90:1347–1352. https://doi.org/10.1136/jnnp-2018-320199
Jiang J, Liu G, Shi S, Li Y, Li Z (2019) Effects of manual acupuncture combined with donepezil in a mouse model of Alzheimer’s disease. Acupunct Med 37:64–71. https://doi.org/10.1136/acupmed-2016-011310
Kang JY, Park SK, Guo TJ, Ha JS, Lee DS, Kim JM, Lee U, Kim DO, Heo HJ (2016) Reversal of trimethyltin-induced learning and memory deficits by 3,5-dicaffeoylquinic acid. Oxid Med Cell Longev 2016:6981595. https://doi.org/10.1155/2016/6981595
Khurana R, Uversky VN, Nielsen L, Fink AL (2001) Is Congo red an amyloid-specific dye? J Biol Chem 276:22715–22721. https://doi.org/10.1074/jbc.M011499200
Kitazawa M, Cheng D, LaFerla FM (2009) Chronic copper exposure exacerbates both amyloid and tau pathology and selectively dysregulates cdk5 in a mouse model of AD. J Neurochem 108:1550–1560. https://doi.org/10.1111/j.1471-4159.2009.05901.x
Kulkarni UD, Kumari Kamalkishore M, Vittalrao AM, Kumar Siraganahalli Eshwaraiah P (2022) Cognition enhancing abilities of vitamin D, epalrestat and their combination in diabetic rats with and without scopolamine induced amnesia. Cogn Neurodyn 16:483–495. https://doi.org/10.1007/s11571-021-09718-6
Kumar V, Kalita J, Bora HK, Misra UK (2016) Relationship of antioxidant and oxidative stress markers in different organs following copper toxicity in a rat model. Toxicol Appl Pharmacol 293:37–43. https://doi.org/10.1016/j.taap.2016.01.007
Kwon KJ et al (2014) Effects of donepezil, an acetylcholinesterase inhibitor, on neurogenesis in a rat model of vascular dementia. J Neurol Sci 347:66–77. https://doi.org/10.1016/j.jns.2014.09.021
Lee DM et al (2009) Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry 80:722. https://doi.org/10.1136/jnnp.2008.165720
Lin CI, Chang YC, Kao NJ, Lee WJ, Cross TW, Lin SH (2020) 1,25(OH)2D3 alleviates Abeta(25–35)-induced tau hyperphosphorylation, excessive reactive oxygen species, and apoptosis through interplay with glial cell line-derived neurotrophic factor signaling in SH-SY5Y cells. Int J Mol Sci 21. https://doi.org/10.3390/ijms21124215
Medhat E, Rashed L, Abdelgwad M, Aboulhoda BE, Khalifa MM, El-Din SS (2020) Exercise enhances the effectiveness of vitamin D therapy in rats with Alzheimer’s disease: emphasis on oxidative stress and inflammation. Metab Brain Dis 35:111–120. https://doi.org/10.1007/s11011-019-00504-2
Melo JB, Agostinho P, Oliveira CR (2003) Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neurosci Res 45:117–127. https://doi.org/10.1016/s0168-0102(02)00201-8
Nourhashemi F et al (2018) Cross-sectional associations of plasma vitamin D with cerebral beta-amyloid in older adults at risk of dementia. Alzheimers Res Ther 10:43. https://doi.org/10.1186/s13195-018-0371-1
Peng HB, Bukuroshi P, Durk MR, Grootendorst P, Yan X, Pan SR, de Lannoy IAM, Pang KS (2021) Impact of age, hypercholesterolemia, and the vitamin D receptor on brain endogenous beta-amyloid peptide accumulation in mice. Biopharm Drug Dispos 42:372–388. https://doi.org/10.1002/bdd.2297
Pogge E (2010) Vitamin D and Alzheimer’s disease: is there a link? The Consultant Pharmacist® 25:440–450
Porsteinsson AP, Isaacson RS, Knox S, Sabbagh MN, Rubino I (2021) Diagnosis of early Alzheimer’s disease: clinical practice in 2021. J Prev Alzheimers Dis 8:371–386. https://doi.org/10.14283/jpad.2021.23
Przybelski RJ, Binkley NC (2007) Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys 460:202–205. https://doi.org/10.1016/j.abb.2006.12.018
Puchacz E, Stumpf WE, Stachowiak EK, Stachowiak MK (1996) Vitamin D increases expression of the tyrosine hydroxylase gene in adrenal medullary cells. Brain Res Mol Brain Res 36:193–196. https://doi.org/10.1016/0169-328x(95)00314-i
Rajasekhar K, Govindaraju T (2018) Current progress, challenges and future prospects of diagnostic and therapeutic interventions in Alzheimer’s disease. RSC Adv 8:23780–23804. https://doi.org/10.1039/C8RA03620A
Salum E, Kampus P, Zilmer M, Eha J, Butlin M, Avolio AP, Podramagi T, Arend A, Aunapuu M, Kals J (2012) Effect of vitamin D on aortic remodeling in streptozotocin-induced diabetes. Cardiovasc Diabetol 11:58. https://doi.org/10.1186/1475-2840-11-58
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1:a006189. https://doi.org/10.1101/cshperspect.a006189
Smolders J, Damoiseaux J, Menheere P, Hupperts R (2008) Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol 194:7–17. https://doi.org/10.1016/j.jneuroim.2007.11.014
Sonkusare S, Srinivasan K, Kaul C, Ramarao P (2005) Effect of donepezil and lercanidipine on memory impairment induced by intracerebroventricular streptozotocin in rats. Life Sci 77:1–14. https://doi.org/10.1016/j.lfs.2004.10.036
Sonnenberg J, Luine VN, Krey LC, Christakos S (1986) 1,25-Dihydroxyvitamin D3 treatment results in increased choline acetyltransferase activity in specific brain nuclei. Endocrinology 118:1433–1439. https://doi.org/10.1210/endo-118-4-1433
Turunc Bayrakdar E, Uyanikgil Y, Kanit L, Koylu E, Yalcin A (2014) Nicotinamide treatment reduces the levels of oxidative stress, apoptosis, and PARP-1 activity in Abeta(1–42)-induced rat model of Alzheimer’s disease. Free Radic Res 48:146–158. https://doi.org/10.3109/10715762.2013.857018
Yamini P, Ray RS, Chopra K (2018) Vitamin D3 attenuates cognitive deficits and neuroinflammatory responses in ICV-STZ induced sporadic Alzheimer’s disease. Inflammopharmacology 26:39–55. https://doi.org/10.1007/s10787-017-0372-x
Yan L, Wu P, Gao DM, Hu J, Wang Q, Chen NF, Tong SQ, Rao L, Liu J (2019) The impact of vitamin D on cognitive dysfunction in mice with systemic lupus erythematosus. Med Sci Monit 25:4716–4722. https://doi.org/10.12659/MSM.915355
Acknowledgements
Authors would like to acknowledge the support given by the animal unit at the Faculty of Pharmacy, Zagazig University, for using the unit facilities and behavior room facilities.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MM designed the work, the idea of the study and revised the manuscripts, MMH carried out the experimental work, MS carried out the histopathology work, NN shared in the laboratory work and drafted the manuscript, and SIA carried out the statistical work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s00210-023-02690-4"
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elseweidy, M.M., Mahrous, M., Ali, S.I. et al. RETRACTED ARTICLE: Vitamin D alleviates cognitive dysfunction and brain damage induced by copper sulfate intake in experimental rats: focus on its combination with donepezil. Naunyn-Schmiedeberg's Arch Pharmacol 396, 1931–1942 (2023). https://doi.org/10.1007/s00210-023-02449-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02449-x