Abstract
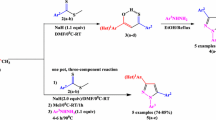
Four novel 3-Aryl -1-(pyridin-4-yl)benzo[4,5]imidazo[1,2-d][1,2,4]- triazin-4(3H)-ones derivatives (C1 to C4) have been designed, synthesized, and evaluated for their anticancer activity. The structure of compounds was characterized by IR,1H NMR, 13C NMR and high-resolution mass (HRMS). The crystal structures of C1, C2 and C4 were previously determined by single-crystal X-ray analysis.
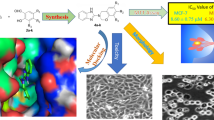
The results from docking experiments with EGFR suggested the binding of the compounds at the active site of EGFR. The new compounds exhibited different levels of cytotoxicity against HCC1937 and MCF7 breast cancer cells. Results of the MTT assay identified C3 as the most cytotoxic of the series against both MCF7 and HCC1937 breast cancer cell lines with IC50 values of 36.4 and 48.2 µM, respectively. In addition to its ability to inhibit cell growth and colony formation ability, C3 also inhibited breast cancer cell migration. Western blotting results showed that C3 treatment inhibited EGFR signaling and induced cell cycle arrest and apoptosis as indicated by the low level of p-EGFR and p-AKT and the increasing levels of p53, p21 and cleaved PARP. Our work represents a promising starting point for the development of a new series of compounds targeting cancer cells.
Graphical abstract








Similar content being viewed by others
Availability of data and materials
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
References
AboulWafa OM, Daabees HMG, Badawi WA (2020) 2-Anilinopyrimidine derivatives: Design, synthesis, in vitro anti-proliferative activity, EGFR and ARO inhibitory activity, cell cycle analysis and molecular docking study. Bioorg Chem 99:103798. https://doi.org/10.1016/j.bioorg.2020.103798
Abraham AG, O’Neill E (2014) PI3K/Akt-mediated regulation of p53 in cancer. Biochem Soc Trans 42:798–803. https://doi.org/10.1042/BST20140070
Abu Thaher B, Koch P, Schollmeyer D, Laufer S (2012) Ethyl 5-amino-3-(pyridin-4-yl)-1-(2,4,6-tri-chlorophenyl)-1H-pyrazole-4- carboxylate dimethyl sulfoxide hemisolvate. Acta Crystallogr Sect E Struct Reports Online 68:o917-8. https://doi.org/10.1107/S1600536812008264
Abu Thaher B, Koch P, Schollmeyer D, Laufer S (2012) 4-(4-Fluorophenyl)-1-(4-nitrophenyl)-3-(pyridin-4-yl)-1H-pyrazol-5-amine. Acta Crystallogr Sect E Struct Reports Online 68:o633. https://doi.org/10.1107/S1600536812004102
Abu Thaher B, Koch P, Schollmeyer D, Laufer S (2012) 4-(4-Fluorophenyl)-3-(pyridin-4-yl)-1-(2,4,6-trichlorophenyl) -1H-pyrazol-5-amine. Acta Crystallogr Sect E Struct Reports Online 68:o2603. https://doi.org/10.1107/S1600536812033569
Abu Thaher B, Koch P, Schollmeyer D, Laufer S (2012) 4-(4-Fluorophenyl)-1-phenyl-3-(pyridin-4-yl)-1H-pyrazol-5-amine. Acta Crystallogr Sect E Struct Reports Online 68:o632. https://doi.org/10.1107/S1600536812004096
Abu Thaher B, Schollmeyer D, Qeshta B, Deigner H-P (2016a) 3-(2,4-Difluorophenyl)-1-(pyridin-4-yl)benzo[4,5]imidazo[1,2- d ][1,2,4]triazin-4(3 H )-one . IUCrData 1:x161380-. https://doi.org/10.1107/S2414314616013808/HB4075ISUP3.CML
Abu Thaher B, Schollmeyer D, Qeshta B, Deigner H-P (2016b) 3-(2,4-Difluorophenyl)-1-(pyridin-4-yl)benzo[4,5]imidazo[1,2- d ][1,2,4]triazin-4(3 H )-one . IUCrData 1:. https://doi.org/10.1107/S2414314616013808
Akhtar MJ, Khan AA, Ali Z et al (2018) Synthesis of stable benzimidazole derivatives bearing pyrazole as anticancer and EGFR receptor inhibitors. Bioorg Chem 78:158–169. https://doi.org/10.1016/j.bioorg.2018.03.002
Aliwaini S, Swarts AJAJAJ, Blanckenberg A et al (2013) A novel binuclear palladacycle complex inhibits melanoma growth in vitro and in vivo through apoptosis and autophagy. Biochem Pharmacol 86:1650–1662. https://doi.org/10.1016/j.bcp.2013.09.020
Aliwaini S, Thaher BA, Al-masri I et al (2021) Design, synthesis and biological evaluation of novel pyrazolo[1,2,4]triazolopyrimidine derivatives as potential anticancer agents. Molecules 26:4065. https://doi.org/10.3390/molecules26134065
Aliwaini S, Thaher BA, Al-masri I et al (2021) Design, Synthesis and Biological Evaluation of Novel Pyrazolo[1,2,4]triazolopyrimidine Derivatives as Potential Anticancer Agents. Mol 26:4065. https://doi.org/10.3390/MOLECULES26134065
Altaher A, Adris M, Aliwaini S et al (2022) The Anticancer Effects of Novel Imidazo[1,2-a]Pyridine Compounds against HCC1937 Breast Cancer Cells. Asian Pacific J Cancer Prev 23:2943–2951. https://doi.org/10.31557/apjcp.2022.23.9.2943
Burnet MW, Thaher BA, Jan Ehlert Kubbutat M, Schaechtele C, Totzke F (2016) (12) United States Patent. 2:1–28
Celik İ, Ayhan-Kılcıgil G, Guven B et al (2019) Design, synthesis and docking studies of benzimidazole derivatives as potential EGFR inhibitors. Eur J Med Chem 173:240–249. https://doi.org/10.1016/j.ejmech.2019.04.012
Choi W, Park SY, Lee Y et al (2021) The Clinical Impact of Capmatinib in the Treatment of Advanced Non-Small Cell Lung Cancer with MET Exon 14 Skipping Mutation or Gene Amplification. Cancer Res Treat 53:1024–1032. https://doi.org/10.4143/crt.2020.1331
Dong G, Jiang Y, Zhang F et al (2022) Recent updates on 1,2,3-, 1,2,4-, and 1,3,5-triazine hybrids (2017–present): The anticancer activity, structure–activity relationships, and mechanisms of action. Arch Pharm (Weinheim). https://doi.org/10.1002/ardp.202200479
DS visualizer (2022) Accelrys Discovery Studio Visualizer 3.0 Download (Free) - DiscoveryStudio25.exe. https://accelrys-discovery-studio-visualizer.software.informer.com/3.0/. Accessed 24 Mar 2020
Gaber AA, Bayoumi AH, El-morsy AM et al (2018) Design, synthesis and anticancer evaluation of 1H-pyrazolo[3,4-d]pyrimidine derivatives as potent EGFR WT and EGFR T790M inhibitors and apoptosis inducers. Bioorg Chem 80:375–395. https://doi.org/10.1016/j.bioorg.2018.06.017
Gellis A, Kovacic H, Boufatah N, Vanelle P (2008) Synthesis and cytotoxicity evaluation of some benzimidazole-4,7-diones as bioreductive anticancer agents. Eur J Med Chem 43:1858–1864. https://doi.org/10.1016/J.EJMECH.2007.11.020
Guo H, Diao Q-P (2020) 1,3,5-Triazine-azole Hybrids and their Anticancer Activity. Curr Top Med Chem 20:1481–1492. https://doi.org/10.2174/1568026620666200310122741
Hashem HE, Amr AEGE, Nossier ES et al (2022) New Benzimidazole-, 1,2,4-Triazole-, and 1,3,5-Triazine-Based Derivatives as Potential EGFRWTand EGFRT790MInhibitors: Microwave-Assisted Synthesis, Anticancer Evaluation, and Molecular Docking Study. ACS Omega 7:7155–7171. https://doi.org/10.1021/acsomega.1c06836
Jung S, Kim DH, Choi YJ et al (2021) Contribution of p53 in sensitivity to EGFR tyrosine kinase inhibitors in non-small cell lung cancer. Sci Rep 11:1–10. https://doi.org/10.1038/s41598-021-99267-z
Kayki G, Zinnet M, Aksoyalp S et al (2022) A year in pharmacology : new drugs approved by the US Food and Drug Administration in 2021. Naunyn Schmiedebergs Arch Pharmacol 395:867–885. https://doi.org/10.1007/s00210-022-02250-2
Krause M, Foks H, Gobis K (2017) Pharmacological potential and synthetic approaches of imidazo[4,5-b]pyridine and imidazo[4,5-c]pyridine derivatives. Molecules 22:399. https://doi.org/10.3390/molecules22030399
Kushwaha N, Sharma CS (2020) The Chemistry of Triazine Isomers: Structures, Reactions, Synthesis and Applications. Mini-Reviews Med Chem 20:2104–2122. https://doi.org/10.2174/1389557520666200729160720
Liang Q, Wang J, Zhao L et al (2021) Recent advances of dual FGFR inhibitors as a novel therapy for cancer. Eur J Med Chem 214:113205. https://doi.org/10.1016/j.ejmech.2021.113205
Loureiro DRP, Soares JX, Costa JC et al (2019) Structures, Activities and Drug-Likeness of Anti-Infective Xanthone Derivatives Isolated from the Marine Environment: A Review. Mol 24:243. https://doi.org/10.3390/MOLECULES24020243
Majeed Ganai A, Khan Pathan T, Hampannavar GA et al (2021) Recent Advances on the s-Triazine Scaffold with Emphasis on Synthesis, Structure-Activity and Pharmacological Aspects: A Concise Review. ChemistrySelect 6:1616–1660. https://doi.org/10.1002/slct.202004591
MarvinSketch16.10.24 (2016) ChemAxon - Software Solutions and Services for Chemistry & Biology
Nawaz F, Alam O, Perwez A et al (2020) 3′-(4-(Benzyloxy)phenyl)-1′-phenyl-5-(heteroaryl/aryl)-3,4-dihydro-1′H,2H-[3,4′-bipyrazole]-2-carboxamides as EGFR kinase inhibitors: Synthesis, anticancer evaluation, and molecular docking studies. Arch Pharm (Weinheim) 353:1900262. https://doi.org/10.1002/ardp.201900262
OEDocking (2021) OEDocking Software | Molecular Docking Tools | Fred Docking. https://www.eyesopen.com/oedocking. Accessed 24 Mar 2020
OMEGA (Version 2.3.2) (2020) OMEGA 4.2.1.2: OpenEye Scientific Software, Santa Fe
Peng YH, Shiao HY, Tu CH et al (2013) Protein kinase inhibitor design by targeting the Asp-Phe-Gly (DFG) motif: The role of the DFG motif in the design of epidermal growth factor receptor inhibitors. J Med Chem 56:3889–3903
Peterson EA, Andrews PS, Be X et al (2011) Discovery of triazine-benzimidazoles as selective inhibitors of mTOR. Bioorganic Med Chem Lett 21:2064–2070. https://doi.org/10.1016/j.bmcl.2011.02.007
Refaat HM (2010) Synthesis and anticancer activity of some novel 2-substituted benzimidazole derivatives. Eur J Med Chem 45:2949–2956. https://doi.org/10.1016/J.EJMECH.2010.03.022
Sahin Z, Biltekin SN, Ozansoy M et al (2022) Synthesis and in vitro antitumor activities of novel thioamide substituted piperazinyl-1,2,4-triazines. J Heterocycl Chem 59:1333–1340. https://doi.org/10.1002/jhet.4470
Schuler M, Berardi R, Lim WT et al (2020) Molecular correlates of response to capmatinib in advanced non-small-cell lung cancer: clinical and biomarker results from a phase I trial. Ann Oncol 31:789–797. https://doi.org/10.1016/j.annonc.2020.03.293
Seifert R (2019) Basic Knowledge of Pharmacology
Sette G, Salvati V, Mottolese M et al (2015) Tyr1068-phosphorylated epidermal growth factor receptor (EGFR) predicts cancer stem cell targeting by erlotinib in preclinical models of wild-type EGFR lung cancer. Cell Death Dis 6:e1850. https://doi.org/10.1038/cddis.2015.217
Sharma P, Larosa C, Antwi J et al (2021) Imidazoles as potential anticancer agents: An update on recent studies. Molecules 26:4213. https://doi.org/10.3390/molecules26144213
Sherbiny FF, Bayoumi AH, El-Morsy AM et al (2021) Design, Synthesis, biological Evaluation, and molecular docking studies of novel Pyrazolo[3,4-d]Pyrimidine derivative scaffolds as potent EGFR inhibitors and cell apoptosis inducers. Bioorg Chem 116:105325. https://doi.org/10.1016/j.bioorg.2021.105325
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30. https://doi.org/10.3322/caac.21590
Singla P, Luxami V, Paul K (2015) Triazine-benzimidazole hybrids: Anticancer activity, DNA interaction and dihydrofolate reductase inhibitors. Bioorganic Med Chem 23:1691–1700. https://doi.org/10.1016/j.bmc.2015.03.012
Sivaramakarthikeyan R, Iniyaval S, Saravanan V et al (2020) Molecular Hybrids Integrated with Benzimidazole and Pyrazole Structural Motifs: Design, Synthesis, Biological Evaluation, and Molecular Docking Studies. ACS Omega 5:10089–10098. https://doi.org/10.1021/acsomega.0c00630
Stec MM, Andrews KL, Bo Y et al (2015) The imidazo[1,2-a]pyridine ring system as a scaffold for potent dual phosphoinositide-3-kinase (PI3K)/mammalian target of rapamycin (mTOR) inhibitors. Bioorganic Med Chem Lett 25:4136–4142. https://doi.org/10.1016/j.bmcl.2015.08.016
Tan L, Zhang J, Wang Y et al (2022) Development of Dual Inhibitors Targeting Epidermal Growth Factor Receptor in Cancer Therapy. J Med Chem 65:5149–5183. https://doi.org/10.1021/ACS.JMEDCHEM.1C01714/ASSET/IMAGES/MEDIUM/JM1C01714_0024.GIF
Thaher BA, Arnsmann M, Totzke F et al (2012) Tri- and tetrasubstituted pyrazole derivates: Regioisomerism switches activity from p38MAP kinase to important cancer kinases. J Med Chem 55:961–965. https://doi.org/10.1021/JM201391U/SUPPL_FILE/JM201391U_SI_001.PDF
Thomas HD, Calabrese CR, Batey MA et al (2007) Preclinical selection of a novel poly(ADP-ribose) polymerase inhibitor for clinical trial. Mol Cancer Ther 6:945–956. https://doi.org/10.1158/1535-7163.MCT-06-0552
Veber DF, Johnson SR, Cheng HY et al (2002) Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem 45:2615–2623. https://doi.org/10.1021/JM020017N/SUPPL_FILE/JM020017N_S.PDF
Wahedy K, Thaher BA, Schollmeyer D et al (2017) Crystal structures of pure 3-(4-bromo-2-chlorophenyl)-1-(pyridin-4-yl)benzo[4,5]imidazo-[1,2-d][1,2,4]triazin-4(3H)-one and contaminated with 3-(4-bromophenyl)-1-(pyridin-4-yl)benzo-[4,5]imidazo[1,2-d][1,2,4]triazin-4(3H)-one. Acta Crystallogr Sect E Crystallogr Commun 73:1341–1343
Yuan X, Yang Q, Liu T et al (2019) Design, synthesis and in vitro evaluation of 6-amide-2-aryl benzoxazole/benzimidazole derivatives against tumor cells by inhibiting VEGFR-2 kinase. Eur J Med Chem 179:147–165. https://doi.org/10.1016/j.ejmech.2019.06.054
Zhang H, Berezov A, Wang Q et al (2007) Review series ErbB receptors : from oncogenes to targeted cancer therapies. Molecules 117:2051–2058. https://doi.org/10.1172/JCI32278.The
Zhang J, Che J, Luo X et al (2022) Structural Feature Analyzation Strategies toward Discovery of Orally Bioavailable PROTACs of Bruton’s Tyrosine Kinase for the Treatment of Lymphoma. J Med Chem 65:9096–125. https://doi.org/10.1021/acs.jmedchem.2c00324
Zhao YH, Abraham MH, Le J et al (2002) (2002) Rate-Limited Steps of Human Oral Absorption and QSAR Studies. Pharm Res 1910(19):1446–1457. https://doi.org/10.1023/A:1020444330011
Acknowledgments
Bassam Abu Thaher would like to thank by the Palestinian Research Council (Ramallah) for funding and Alexander von Humboldt Foundation (AvH) for funding his short visit, in 2022.
Funding
This work was supported by Palestinian Research Council (Ramallah) and Alexander von Humboldt Foundation (AvH) for funding his short visit, in 2022.
Author information
Authors and Affiliations
Contributions
B.A, B.Q and R.M prepared the compounds. C.J and H.D supervised the chemical design and synthesis. I. A and K.W performed the modeling. S.I wrote the main manuscript and supervised the biological work.S.kh, I.A, A.M, and A. I performed biological experiments. All authors reviewed the manuscript. The authors declare that all data were generated in-house and that no paper mill was used
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no financial AND non-financial interests conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thaher, B.A., Al-Masri, I., Wahedy, K. et al. Synthesis and bioassay of 3-Aryl -1-(pyridin-4-yl)benzo[4,5]imidazo[1,2-d][1,2,4]- triazin-4(3H)-ones as anti-cancer agents. Naunyn-Schmiedeberg's Arch Pharmacol 396, 1797–1810 (2023). https://doi.org/10.1007/s00210-023-02433-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02433-5