Abstract
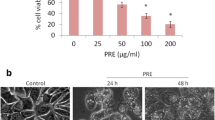
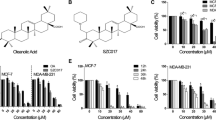
Unfolded protein response (UPR) is involved in breast cancer (BC) progression and drug resistance. Many natural products (NPs) could modulate UPR and used for therapeutic purposes. Herein, we aimed to investigate the molecular mechanism of Cycloart-23E-ene-3β, 25-diol (Cycloart-E25), cytotoxicity, as a NP extracted from Euphorbia macrostegia and focused on endoplasmic-reticulum stress (ERS) and UPR signaling pathways. Reactive oxygen species (ROS) were probed by DCFDA fluorescence dye. Apoptosis was assayed by annexin V/propidium iodide (PI), immunoblotting of anti- and proapoptotic, Bcl-2 and Bax proteins, and mitochondrial transmembrane potential (ΔΨm) changes. Thioflavin T (ThT) staining and immunoblotting of UPR signaling components (CHOP, PERK, ATF6, BiP, and XBP1) were recruited for the assessment of ERS. Our results indicated that Cycloart-E25 noticeably increases ROS levels in both MB-231 MDA-MB-231 and MCF-7 cell lines, p>0.05. Flow cytometry assessments revealed an increase in the cell population undergoing apoptosis. Also, the Bax/Bcl-2 ratio increased in a dose-dependent manner following Cycloart-E25 treatment, significantly, p>0.05. Mitochondrial involvement could be deduced by significant decreases in ΔΨm, p>0.05. Cycloart-E25 potently induces protein aggregation and upregulated CHOP, PERK, ATF6, BiP, and XBP1 factors in both MDA-MB-231 MB-231 and MCF-7 cell lines, indicating the involvement of ERS in Cycloart-E25-mediated apoptosis. In conclusion, Cycloart-E25 increased the accumulation of misfolded proteins and upregulated UPR components. Therefore, induction of ERS may be involved in the trigger of apoptosis in BC cell lines. Cycloart-E25 induced apoptosis in breast cancer cell lines through ERS. More assessments are needed to confirm its in vivo anti-tumoral effects.








Similar content being viewed by others
DATA availability
All data and materials were presented in the “Result” section.
Abbreviations
- BC:
-
Breast cancer
- UPR:
-
Unfolded protein response
- ERS:
-
Endoplasmic-reticulum stress
- PERK:
-
Protein kinase RNA-activated (PKR)-like ER kinase
- ATF6:
-
Activating transcription factor 6
- BiP:
-
Binding immunoglobulin protein
- XBP1:
-
x-box-binding protein 1
- CHOP:
-
CCAAT-enhancer-binding protein homologous protein ()
- Cycloart-E25:
-
Cycloart-23E-ene-3β, 25-diol
- MTT:
-
3-(4, 5-dimethylthiazol-2- yl)-2, 5-diphenyl tetrazolium bromide
- PI:
-
Propidium iodide
- DCFDA:
-
2′,7′-dichlorofluorescein diacetate
- ΔΨm:
-
Mitochondrial transmembrane potential
- ThT:
-
ThioflavinT
References
Almanza A, Carlesso A, Chintha C, Creedican S, Doultsinos D, Leuzzi B, Luís A, McCarthy N, Montibeller L, More S (2019) Endoplasmic reticulum stress signalling–from basic mechanisms to clinical applications. FEBS J 286(2):241–278
Almeida A, Dong L, Appendino G, Bak S (2020) Plant triterpenoids with bond-missing skeletons: biogenesis, distribution and bioactivity. Nat Prod Rep 37(9):1207–1228
Amen, O. M., S. D. Sarker, R. Ghildyal and A. Arya (2019). “Endoplasmic reticulum stress activates unfolded protein response signaling and mediates inflammation, obesity, and cardiac dysfunction: therapeutic and molecular approach.” Frontiers in pharmacology: 977.
Baniadam S, Rahiminejad MR, Ghannadian M, Saeidi H, Ayatollahi AM, Aghaei M (2014) Cycloartane triterpenoids from Euphorbia macrostegia with their cytotoxicity against MDA-MB48 and MCF-7 cancer cell lines. IJPR 13(1):135
Chen H, Yang J, Yang Y, Zhang J, Xu Y, Lu X (2021) The natural products and extracts: anti-triple-negative breast cancer in vitro. Chem Biodivers 18(7):e2001047
Chudzik M, Korzonek-Szlacheta I, Król W (2015) Triterpenes as potentially cytotoxic compounds. Molecules 20(1):1610–1625
Cullinan SB, Diehl JA (2004) PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem 279(19):20108–20117
Daneshforouz A, Nazemi S, Gholami O, Kafami M, Amin B (2021) The cytotoxicity and apoptotic effects of verbascoside on breast cancer 4T1 cell line. BMC Pharmacol Toxicol 22(1):1–9
Denizli N, Horo I, Gülcemal D, Masullo M, Festa M, Capasso A, Koz Ö, Piacente S, Alankuş-Çalışkan Ö (2014) Cycloartane glycosides from Astragalus plumosus var. krugianus and evaluation of their antioxidant potential. Fitoterapia 92:211–218
Du Y, Cai Z, Zhou G, Liang W, Man Q, Wang W (2022) Perfluorooctanoic acid exposure increases both proliferation and apoptosis of human placental trophoblast cells mediated by ER stress-induced ROS or UPR pathways. Ecotoxicol Environ Saf 236:113508
Fang ZZ, Nian Y, Li W, Wu JJ, Ge GB, Dong PP, Zhang YY, Qiu MH, Liu L, Yang L (2011) Cycloartane triterpenoids from Cimicifuga yunnanensis induce apoptosis of breast cancer cells (MCF7) via p53-dependent mitochondrial signaling pathway. Phytother Res 25(1):17–24
Jafri A, Bano S, Rais J, Khan F, Shivnath N, Sharma A, Arshad M (2019) Phytochemical screening of Sterculia foetida seed extract for anti-oxidant, anti-microbial activity, and detection of apoptosis through reactive oxygen species (ROS) generation, mitochondrial membrane potential (MMP) decrease, and nuclear fragmentation in human osteosarcoma cells. J Histotechnol 42(2):68–79
Kemboi D, Peter X, Langat M, Tembu J (2020) A review of the ethnomedicinal uses, biological activities, and triterpenoids of Euphorbia species. Molecules 25(17):4019
Kim C, Kim B (2018) Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: a review. Nutrients 10(8):1021
Knupp J, Arvan P, Chang A (2019) Increased mitochondrial respiration promotes survival from endoplasmic reticulum stress. Cell Death Differ 26(3):487–501
Ling T, Boyd L, Rivas F (2022) Triterpenoids as reactive oxygen species modulators of cell fate. Chem Res Toxicol 35(4):569–584
Ma B, Zhang H, Wang Y, Zhao A, Zhu Z, Bao X, Sun Y, Li L, Zhang Q (2018) Corosolic acid, a natural triterpenoid, induces ER stress-dependent apoptosis in human castration resistant prostate cancer cells via activation of IRE-1/JNK, PERK/CHOP and TRIB3. J Exp Clin Cancer Res 37(1):1–16
Majolo F, L. K. d. O. B. Delwing, D. J. Marmitt, I. C. Bustamante-Filho and M. I. Goettert, (2019) Medicinal plants and bioactive natural compounds for cancer treatment: important advances for drug discovery. Phytochem Lett 31:196–207
Mamadalieva NZ, Youssef FS, Hussain H, Zengin G, Mollica A, Al Musayeib NM, Ashour ML, Westermann B, Wessjohann LA (2021) Validation of the antioxidant and enzyme inhibitory potential of selected triterpenes using in vitro and in silico studies, and the evaluation of their ADMET properties. Molecules 26(21):6331
Martucciello S, Masullo M, Cerulli A, Piacente S (2020) Natural products targeting ER stress, and the functional link to mitochondria. Intl J Mole Sci 21(6):1905
Nishitoh H (2012) CHOP is a multifunctional transcription factor in the ER stress response. J Biochem 151(3):217–219
Rehan M, Mir SA (2020) Structural diversity, natural sources, and pharmacological potential of plant-based saponins with special focus on anticancer activity: a review. Med Chem Res 29(10):1707–1722
Ren Y, Kinghorn AD (2019) Natural product triterpenoids and their semi-synthetic derivatives with potential anticancer activity. Planta Medica 85(11/12):802–814
Sancho-Garnier, H. and M. Colonna (2019). “Breast cancer epidemiology. Presse Medicale (Paris, France: 1983) 48(10): 1076-1084.
Santamaría PG, Mazón MJ, Eraso P, Portillo F (2019) UPR: an upstream signal to EMT induction in cancer. J Clin Med 8(5):624
Shahrestanaki MK, Arasi FP, Aghaei M (2019) Adenosine protects pancreatic beta cells against apoptosis induced by endoplasmic reticulum stress. J Cell Biochem 120(5):7759–7770
Shi J-M, Bai L-L, Zhang D-M, Yiu A, Yin Z-Q, Han W-L, Liu J-S, Li Y, Fu D-Y, Ye W-C (2013) Saxifragifolin D induces the interplay between apoptosis and autophagy in breast cancer cells through ROS-dependent endoplasmic reticulum stress. Biochem Pharmacol 85(7):913–926
Sisinni L, Pietrafesa M, Lepore S, Maddalena F, Condelli V, Esposito F, Landriscina M (2019) Endoplasmic reticulum stress and unfolded protein response in breast cancer: the balance between apoptosis and autophagy and its role in drug resistance. Intl J Mole Sci 20(4):857
Siwecka N, Rozpędek W, Pytel D, Wawrzynkiewicz A, Dziki A, Dziki Ł, Diehl JA, Majsterek I (2019) Dual role of endoplasmic reticulum stress-mediated unfolded protein response signaling pathway in carcinogenesis. Intl J Mole Sci 20(18):4354
Funding
Financial support was made by Isfahan University of Medical Sciences Grants (195001 and 393064).
Author information
Authors and Affiliations
Contributions
Mohammad Keyvaloo Shahrestanki and Abdollah Mirjani performed the experimental part and prepared the drafts of the manuscript. Mahmoud Aghaei and Mustafa Ghanadian designed the research, finalized the manuscript, and provided funds.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shahrestanaki, M.K., Mirjani, A., Ghanadian, M. et al. Cycloartane triterpenoid from Euphorbia macrostegia modulates ER stress signaling pathways to induce apoptosis in MDA-MB231 and MCF-7 breast cancer cell lines. Naunyn-Schmiedeberg's Arch Pharmacol 396, 1749–1758 (2023). https://doi.org/10.1007/s00210-023-02426-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02426-4