Abstract
Buyang Huanwu decoction, a classic traditional Chinese prescription, has been used to prevent and treat stroke for hundreds of years. An increasing number of the laboratory research on Buyang Huanwu decoction used in treating cerebral ischemia–reperfusion injury have been published recently. However, the problem of methodological and reporting quality of some studies is lack of assessment. This study aims to evaluate the methodological and reporting quality of the research on Buyang Huanwu decoction against experimental cerebral ischemia–reperfusion injury. A comprehensive search on six databases was performed. Two researchers independently screened the literature considering the eligibility criteria. Methodological and reporting quality of the included studies were evaluated by the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk-of-bias tool and Animal Research: Reporting of In Vivo Experiments (ARRIVE) guideline. Forty-five studies met the inclusion criteria. No study achieved a decent overall rating in using the SYRCLE tool (percentage of items with “low risk” ≥ 50%). Of the 22 items on the SYRCLE tool, only 7 items (31.82%) were rated as “low risk” in more than 50% of the included studies. Of the 39 items of ARRIVE guideline, 14 (35.9%) items were rated as “yes” in more than 50% of the included studies. The methodological and reporting quality of Buyang Huanwu decoction for experimental cerebral ischemia–reperfusion injury was substandard, which needed to be further improved. The limitations should be addressed when planning similar studies in the future. Additionally, these findings provided evidence-based guidance for future preclinical studies evaluating the efficacy of Buyang Huanwu decoction in the treatment of cerebral ischemia–reperfusion injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preclinical research, particularly important to biomedical research, forms the foundation on which future studies are built. The exciting and new ideas it provides will eventually turn into clinical studies and new drugs that provide benefit to humankind. However, some preclinical research is poorly predictive of ultimate success in the clinic owing to the low methodology and reporting quality (Hackam and Redelmeier, 2006; Perel et al. 2007). Indeed, a structured approach or full details in the randomization steps, baseline characteristic balance, blinding procedures, sample size calculations, or sufficient reporting of an experiment allow for reproducibility of the findings. However, an increasing number of evidence showed highly inadequate methodological and reporting quality upon animal research in some scientific publications (Kilkenny et al. 2012). Thus, there is a growing need for valid, efficient, and easy scoring scales and systematic assessment to provide rigorous scientific methods and rate the quality of animal studies. At present, a few studies have assessed the quality of experimental methods and reports by evaluating compliance with various assessment tools (Fabian-Jessing et al. 2018). The Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE), based on the Cochrane Collaboration risk-of-bias (RoB) tool, is an assessment instrument to evaluate the risk of bias and the methodological quality of animal studies (Hooijmans et al. 2014). The Animal Research: Reporting In Vivo Experiments (ARRIVE), a comprehensive set of guidelines for animal research, provides format and content of details relating to animals in a typical scientific report (Kilkenny et al. 2012). Thus, adherence to the two comprehensive sets of rules for inclusion of detail that is consistent across all articles has many advantages (McGrath et al. 2010).
Worldwide, stroke is an important cause of disability and mortality. The burden of stroke has increased substantially over the past few decades due to population growth and aging as well as the increased prevalence of modifiable stroke risk factors, especially in developing countries (Katan and Luft, 2018). At present, there are three different types of strokes: Ischemic strokes, hemorrhagic strokes, and transient ischemic attacks, among which ischemic strokes account for about 87% of all strokes (Virani et al. 2020). Ischemic stroke is characterized by the sudden loss of blood circulation to an area of the brain, typically in a vascular territory, resulting in a corresponding loss of neurologic function (Virani et al. 2020). Currently, FDA-approved drug for ischemic stroke is the recombinant tissue plasminogen activator (r-tPA), a fibrinolytic agent, which is effective if applied within 3 h, but no longer than 4.5 h, after symptom onset (Rabinstein, 2017). However, cerebral ischemia–reperfusion injury (CIRI) following the application of r-tPA sometimes will lead to secondary injury to the brain tissue. The short therapeutic time window and CIRI limited the benefit of r-tPA for large clot burden, and hence, research is ongoing to find more effective and safer reperfusion therapy, as well as focusing on refinement of patient selection for acute reperfusion treatment (Fukuta et al. 2017).
According to the theory of traditional Chinese medicine (TCM), the primary pathological process of ischemic stroke is Qi deficiency and blood stasis, and Qi is an important concept in the theory of TCM, which is a vital energy that can invigorate the body and promote blood circulation and meridian circulation (Li et al. 2014; Tan et al. 2016; Zhao et al. 2012). Buyang Huanwu decoction (BHD), a classic traditional Chinese prescription invented by the Chinese well-known herbalist Wang Qing-ren (AD 1768–1831) for the treatment of ischemic stroke with Qi deficiency and blood stasis, exhibits the efficacy of replenishing Qi and activating blood circulation according to the theory of TCM. It has been utilized clinically and showed significant preventive and therapeutic effects for ischemic stroke and stroke-induced disability for more than 190 years in China and some other Asian countries (Wei et al. 2013). Recently, increasing clinical evidence about BHD application have showed significant improvement in neural functions and symptoms for ischemic stroke (Han et al. 2018; Hao et al. 2012; Jiang et al. 2020; Li et al. 2014), while the underlying mechanisms remain indistinct. BHD consists of seven kinds of Chinese medicinal materials: Radix Astragali, Radix Paeoniae Rubra, Radix Angelicae Sinensis, Rhizoma Ligustici Chuanxiong, Flos Carthami, Semen Persicae, and Pheretima Aspergillum (Table 1) (Cui et al. 2015). Growing experimental studies reported that BHD was beneficial for cerebral ischemia and CIRI, suggesting that BHD may be a prospective therapy that could decrease infarct volume and ameliorate neurological impairment (Cai et al. 2007; Chen et al. 2020a, b, 2019). There are basic methodology and reporting practices in the laboratory study upon BHD treatment against CIRI that are sub-optimal, and it is likely to be affecting the validity and replicability of research.
Thus, the aim of this study was to assess the methodological and reporting quality of experimental researches concerning BHD treatment for CIRI with the SYRCLE tool and ARRIVE guideline, respectively, to provide valuable insights for future studies and support the development of methodological and reporting guidance.
Methods
Search strategies
Studies of BHD in animal models of CIRI were identified from PubMed, Embase, China National Knowledge Infrastructure (CNKI), VIP Database for Chinese Technical Periodicals (VIP), China Biology Medicine Database (CBM), and Wan Fang Data until November 23, 2022. Our search strategy included the following words and phrases: “Buyang Huanwu” OR “Bu yang Huan wu” OR “Bu-yang Huan-wu” AND “cerebral ischemia–reperfusion.”
Inclusion criteria and data extraction
An eligible study had to meet all of the following criteria: (1) The retrieval date was from January 1, 2015 to November 23, 2022; (2) only journal articles were selected; (3) experimental models of CIRI were induced in rats; (4) Buyang Huanwu decoction was used as treatment; and (5) articles published in Chinese or English languages were included. Studies were excluded if they were in vitro studies, clinical articles, review comments, publication without full text, duplicated researches, and language other than English or Chinese. The following information was extracted using a predesigned data extraction form from each eligible study: first author, year of publication, strain, sex, weight, anesthetic, method of establishing the model, and effects of BHD. All the included studies were checked for the consistency separately by two assessors (XY Chen, T Yang), and disagreement would be settled by discussion and a third assessor (ZG Mei).
Methodological and reporting quality evaluation
Two assessors (XY Chen, T Yang) were trained to assess the methodological and reporting quality by experienced assessors before the assessment started. Each reviewer independently assessed the quality of each study. Methodological quality evaluation was assessed using the SYRCLE tool. According to the SYRCLE tool, each item was assigned one of three responses: “low risk, unclear or high risk.” Reporting quality evaluation was performed using the ARRIVE guideline. According to the satisfaction degree of item reporting requirements, it can be divided into “yes,” “partial yes,” and “no.” In the case of a discrepancy, each reviewer provided reasoning for the judgment and disagreements were solved by discussion. If necessary, the third assessor (ZG Mei) was involved in judgment.
Data analysis
Microsoft Excel 2016 was used for the descriptive statistical analysis, and summary statistics were given percentages. Kappa test was performed by utilizing SPSS 25.0 (IBM Corp., Armonk, NY, USA). The kappa index was used to measure the inter-rater reliability between the two assessors for the SYRCLE tool and ARRIVE guideline. A kappa index less than 0.4 suggested poor agreements, 0.4 to 0.75 suggested fair agreements, and over 0.75 suggested excellent agreements.
Results
Study selection
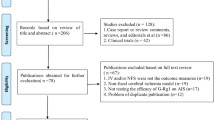
The search strategies yielded 284 records in total. After duplicating retrieval by the NoteExpress database, 114 studies were remained. Among these, 50 records were excluded due to failing to meet the inclusion criteria after screening the titles and abstracts. The full texts of the remaining 64 records were examined for further assessment. And, 19 studies were discarded because of duplicated publication and improper indices. Finally, we included 45 studies in this overview. A flow chart describing the systematic search and study selection process is shown in Fig. 1.
Basic characteristics of included studies
Among the included studies, about 80% of studies were published in Chinese-language journals, and the remaining 9 studies were in English language. In terms of strain and sex of rats, 35 studies used male of Sprague Dawley, 1 study utilized male of Wistar, and 4 studies used female and male of Sprague Dawley. Five studies used Sprague Dawley without indicating sex of rats. The weight of rats varied from 150 to 320 g in each study. There were 27 studies anesthetized rats with chloral hydrate, 8 studies with pentobarbital sodium, 4 studies with isoflurane, 1 study with 3% amobarbital sodium, 1 study with Zoletil 50 and xylazine, and the remaining 4 studies not listed in the article. Different methods of modeling were performed, including middle cerebral artery occlusion, 4-vessel occlusion, carotid artery drainage, and bilateral carotid artery occlusion. Different outcome indexes were observed, and the most frequently used indicator was neurological severity score (in 19 studies); others included cerebral infarct size, cerebral edema volume, Bcl-2, Bax, VEGF, AKT, p-AKT, IL-6, SOD, and so on. The main characteristics of including studies are displayed in Table 2.
Methodological quality of included studies
General compliance with the SYRCLE tool was incomplete. No study achieved a decent overall rating (percentage of items with “low risk” ≥ 50%) with the SYRCLE tool. Therefore, the items of all the studies were poorly evaluated. Among all the 22 items, merely 2 items about the published report included all expected outcomes, which was rated as low risk bias in all articles. Eleven studies (24%) described a random component in the sequence generation process. One study (2%) kept the distribution of relevant baseline characteristics balanced for the intervention and control groups. Sixteen studies (36%) induced the disease before randomization of the intervention, and twenty-eight studies (62%) mentioned the outcome was not influenced by not randomly housing the animals. When it came to whether the outcome assessor was blinding, two studies (4%) blinded outcome assessor and judged the outcome was not likely to be influenced by lack of blinding. Twenty-three studies held all animals included in the analysis to ensure adequate outcome data. Only one study reported new animals added to the control and experimental groups to replace dropouts from the original population. There were still 8 items that were poorly evaluated, including the allocation of concealment, housing of animals randomly, implementation of blinding between the caregivers and investigators, selection of animals randomly, planning of a protocol and other problems that could result in a high risk of bias. Overall, only 7 items (31.82%) were rated as “low risk” in more than 50% of the included studies of the 22 items on the SYRCLE tool. The inter-rater reliability was excellent between the two assessors (kappa = 0.94). The details can be found in Table 3 and Fig. 2.
Reporting quality of included studies
A summary of the ARRIVE guideline results is demonstrated in Table 4 and Fig. 3. No study fulfilled all 39 items of ARRIVE guideline. Merely three studies (7%) described the complete ethical statement. Two studies (4%) drew a time chart or flow chart (Fig. 3(6d)), described the procedure implementation place (Fig. 3(7c)), provided complete details of the animals used (Fig. 3(8a)), explained the reason why some animals or data were not included (Fig. 3(15b)), and commented on the study limitations (Fig. 3(18b)). Six studies (13%) reported housing (Fig. 3(9a)), and eight studies (18%) provided adequate husbandry conditions (Fig. 3(9b)). Nine studies (20%) mentioned randomized grouping. Even worse, there were 10 items even achieving a 100% “no,” including the explanation of how and why the animal species and model were used (Fig. 3(3b)), the description of the procedure implementation time (Fig. 3(7b)), welfare-related evaluations, interventions that were carried out throughout the experiment (Fig. 3(9c)), detailed sample size calculation (Fig. 3(10b)), the order in which the animals in the different experimental groups were treated and assessed (Fig. 3(11b)), the unit of analysis for each dataset specially (Fig. 3(13b)), an offer of the baseline data of experimental animals (Fig. 3(14)), details of important adverse events in each group (Fig. 3(17a)), modifications to the experimental protocols (Fig. 3(17b)), and any implications of your experimental methods or findings for the 3Rs (Fig. 3(18c)), while there were 6 items accurately reported, achieving a 100% “yes,” including a title that accurately described the content of the article (Fig. 3(1)); a study design that mentioned experimental unit (Fig. 3(6c)); an experimental procedure that provided precise details of drug formulation and dose, site and route of administration, anesthesia used, surgical procedure, and method of euthanasia (Fig. 3(7a)); a clear definition that the primary and secondary experimental outcomes were assessed (Fig. 3(12)); details of the statistical methods used for each analysis (Fig. 3(13a)); and the reports on the results of each analysis and accuracy of measures (Fig. 3(16)). Overall, in the 39 items of ARRIVE guideline, 14 (35.90%) items were rated as “yes” in more than 50% of the included studies. The inter-rater reliability was excellent between the two assessors (kappa = 0.95).
Discussion
Experimental researches involving animal models play a crucial role in scientific innovation provided that the experiments are designed, performed, interpreted, and reported well (Bezdjian et al. 2018). Hitherto, an increasing number of experimental researches have reported that BHD is beneficial for CIRI. It has been reported that BHD can protect neurons from ischemic injury, reduce infarction volumes, and stimulate neural proliferation (Zhang et al. 2018). BHD has exhibited the profile as a potential target medicine for the treatment of ischemic stroke or CIRI in facilitating the translation of basic science to the clinical application. However, the conclusions and results may be impeded due to the methodological flaws and poor reporting of experimental researches. Hence, this study aims to assess the methodological and reporting quality of experimental research concerning Buyang Huanwu decoction for mitigating CIRI in rats, to provide useful suggestions for the implementation of the future reviewers and researchers. Unfortunately, the results revealed some limitations in the quality of methodology and reporting, suggesting the need for an improvement in quality in the future.
The methodological quality of included studies is assessed using the SYRCLE tool. The SYRCLE RoB tool (Hooijmans et al. 2014), with 10 items and 22 sub-items, is considered to have high reliability and practicability to evaluate the methodological quality of animal experiments. It not only can assess the risk of bias and improve transparency in the animal research process, but also include a comprehensive user guide. As the sample size of most animal experiments is relatively smaller than that of clinical trials, therefore, necessary baseline characteristics and adequate timing of disease induction for animal experimental disease modeling should be determined to reduce baseline imbalance. In our previous experimental research (Mei et al. 2022, 2020; Yang, et al. 2021), we observed that the 90 min and 24 h may be the appropriate lasting time of MCAO and reperfusion in rat stroke model to mimic the clinical injury of cerebral ischemia and reperfusion. Adequate randomization, allocation concealment, and blinding are suggested to be implemented carefully to reduce the risk of selection bias, performance bias, and detection bias. Also, incomplete outcome data should be reported to reduce the risk of attrition bias, including the appropriate imputations and reasons for missing outcome data. Adherence to a well-developed protocol can reduce the risk of reporting bias. However, these items are not well explained because of a database of registered animal research protocols is not yet publicly accessible. In addition to the above, some other sources of bias need to be paid attention including the contamination, inappropriate influence of funders, unit of analysis errors, design-specific risks of bias, and new animals added to the groups to replace dropouts from the original population.
As for the reporting quality of included studies, we assess it by the ARRIVE guideline. The ARRIVE guideline (Kilkenny et al. 2012), with 20 items and 39 sub-items, aims to fill the gap lacking a set of comprehensive animal research reporting guidelines. It involves important information of animal experiments and promotes substantial improvements in methods used in in vivo animal research. The ARRIVE guideline has been endorsed by over 300 research journals around the world in 2014. Nowadays, the ARRIVE guideline 2.0 has been published in 2020 (Percie du Sert et al. 2020). According to this guideline, it is necessary to explain the reason of using the animal species and model and the study’s relevance to human biology. The full detailed description of the experimental procedures, including the implementation time of procedures, the reason for the route of administration, and the drug dose selection, is the key to ensure accurate experimental reproduction. Baseline data of experimental animals is essential for the results to be comparable. The authors should precisely and explicitly record the details of the animals used including source, species, strain, international strain nomenclature, sex, developmental stage, weight, genetic modification status, genotype, health/immune status, and previous procedures. The information of relevant characteristics and health status of animals before treatment or testing can often be tabulated. The risk of overestimating intervention benefits may be induced by inadequate samples. An adequate sample size with enough statistical power can easily detect statistical differences between groups. So, it is necessary to calculate the sample size before the experiment. It is also significant to focus on adverse events in animal researches to determine the pros and cons of an intervention. In addition, the significance of the study is closely relevant to the utilization rate and conversion of the study. It is also necessary to discuss whether and how these study findings can translate into other species or systems.
The results suggested that methodological and reporting quality should be controlled strictly during the design, implementation, interpretation, and report of experimental research. The SYRCLE tool and ARRIVE guideline could be used to assess the whole process of the animal experiment both rigorously and comprehensively. Nowadays, most studies have low methodological and reporting quality. A recent research (Zhang et al. 2019a) shows that Chinese basic medical researchers have a low awareness and use rates of the SYRCLE tool and the ARRIVE guideline, leading to the low quality of animal studies in Chinese journals (Wang et al. 2019). In this review, 80% of studies are published in Chinese-language journals. Therefore, it is necessary to take specific measures to promote and popularize these standards and specifications and to introduce them into guidelines of Chinese domestic journals as soon as possible so as to raise awareness and increase utilization rates of researchers and journal editors.
Although we follow strict procedures in this review, it still has some limitations. Firstly, to some extent, the quality defects of the included studies affect our evaluation results. Secondly, the literatures included in our study are only in Chinese and English languages, which may have a linguistic bias. Thirdly, this is the first time that the evaluation analysts use the SYRCLE tool and ARRIVE guideline. The evaluation of many items involved is inevitably subjective and may have led to bias. Finally, the findings may not be applicable to other traditional Chinese medicine trials due to the interventions merely contained BHD and the species restricted to rats.
Conclusions
BHD has been used increasingly in the preclinical researches to treat CIRI, which seems to be a potential treatment option for alleviating CIRI in patients. However, based on the SYRCLE tool and ARRIVE guideline, the methodological and reporting quality of BHD against CIRI were poor. Our findings will urge journal editors; the researchers, clinicians, and reviewers; or funding agencies to pay more attention to address these deficiencies and strengthen training to meet relevant requirements on methodologies and reporting quality by strictly adopting and adhering to well-developed reporting guidelines: the SYRCLE tool and ARRIVE guideline.
Data availability
All data are available from the first author (Xiangyu Chen) on reasonable request.
Abbreviations
- 5-HT:
-
5-Hydroxytryptamine
- 6-keto-PGF1α:
-
6-Keto prostaglandin F1α
- AMPK:
-
Adenosine monophosphate–activated protein kinase
- APTT:
-
Activated partial thromboplastin time
- Arg-1:
-
Arginase-1
- ARRIVE:
-
Animal Research: Reporting In Vivo Experiments
- ASC:
-
Apoptosis-associated speck-like protein
- ATG12:
-
Autophagy-related gene 12
- Bax:
-
Bcl2-associated X
- BBB:
-
Blood-brain barrier
- Bcl-2:
-
B-cell lymphoma 2
- BDNF:
-
Brain-derived neurotrophic factor
- BHD:
-
Buyang Huanwu decoction
- BrdU:
-
5-Bromo-2′-deoxyuridine
- BWT:
-
Beam walking test
- Caspases:
-
Cysteine aspartase
- CBF:
-
Cerebral blood flow
- CBM:
-
China Biology Medicine Database
- CD206:
-
Cluster of differentiation 206
- CD86:
-
Cluster of differentiation 86
- CIRI:
-
Cerebral ischemia–reperfusion injury
- CNKI:
-
China National Knowledge Infrastructure
- Cx43:
-
Connexin 43
- cyt-c:
-
Cytochrome C
- DCX:
-
Doublecortin on the X chromosome
- Drp1:
-
Dynamin-related protein 1
- EEG:
-
Electroencephalograph
- elF2α:
-
Eukaryotic initiation factor 2α
- Embase:
-
Excerpta Medica Database
- ENaC:
-
Epithelial sodium channel
- FDA:
-
Food and Drug Administration
- FIB:
-
Fibrinogen
- Fis1:
-
Fission 1
- FPR2:
-
Formyl peptide receptor 2
- GAP-43:
-
Growth-associated protein 43
- GFAP:
-
Glial fibrillary acidic protein
- GPX/GSH-Px:
-
Glutathione peroxidase
- GRP78:
-
Glucose-regulated protein 78
- GSH:
-
Glutathione
- GSK:
-
Glycogen synthase kinase
- GST:
-
Glutathione S-transferase
- HIF:
-
Hypoxia-inducible factor
- IFN:
-
Interferon
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- ITGαvβ3:
-
Integrin αvβ3
- IκBα:
-
Inhibitor α of NF-κB
- LC3:
-
Microtubule-associated protein light chain 3
- LDH:
-
Lactate dehydrogenase
- MDA:
-
Malondialdehyde
- MMP:
-
Matrix metalloproteinase
- mRNA:
-
Messenger ribonucleic acid
- mTOR:
-
Mammalian target of rapamycin
- MVD:
-
Microvascular density
- MWM:
-
Morris water maze
- NeuN:
-
Neuronal nuclei
- NF-κB:
-
Nuclear factor-kappa B
- NO:
-
Nitric oxide
- NOX2:
-
Nicotinamide adenine dinucleotide phosphate II (NADPH) oxidase 2
- PAF:
-
Platelet activating factor
- p-AKT:
-
Phosphothreonine kinase
- PDK1:
-
Phosphoinositide-dependent protein kinase 1
- PERK:
-
Protein kinase RNA-like endoplasmic reticulum kinase
- PI3K:
-
Phosphatidylinositol 3-kinase
- PT:
-
Prothrombin time
- PTEN:
-
Phosphatase and tensin homolog
- ROS:
-
Reactive oxygen species
- RvD1:
-
Resolvin D1
- SAB :
-
Spontaneous alternation behavior
- SD:
-
Sprague Dawley
- SIRT1:
-
Sirtuin-1
- SOD:
-
Superoxide dismutase
- SYN:
-
Synaptophysin
- SYRCLE:
-
Systematic Review Centre for Laboratory Animal Experimentation
- TGF-β:
-
Transforming growth factor-β
- TLR4:
-
Toll-like receptor 4
- TNF:
-
Tumor necrosis factor
- TT:
-
Thrombin time
- TUNEL:
-
Terminal-deoxynucleotidyl transferase–mediated nick end labeling
- TXB2:
-
Thromboxane B2
- ULK1:
-
UNC-51-like kinase 1
- VEGF:
-
Vascular endothelial growth factor
- VIP:
-
VIP Database for Chinese Technical Periodicals
- WBV:
-
Whole blood viscosity
- Wnt3a:
-
Wingless-type MMTV integration site family member 3a
- ZO-1:
-
Zonula occluden-1
References
Bezdjian A, Klis SFL, Peters JPM, Grolman W, Stegeman I (2018) Quality of reporting of otorhinolaryngology articles using animal models with the ARRIVE statement. Lab Anim 52(1):79–87
Cai G, Liu B, Liu W, Tan X, Rong J, Chen X, Tong L, Shen J (2007) Buyang Huanwu decoction can improve recovery of neurological function, reduce infarction volume, stimulate neural proliferation and modulate VEGF and Flk1 expressions in transient focal cerebral ischemic rat brains. J Ethnopharmacol 113(2):292–299
Cai J, Zhang JP, Yao H, Zhu H, Huang HY, Xu YL (2015) Influence of Buyang Huanwu Tang on expressions of AKT and phosphorylated-AKT in acute cerebral ischemia-reperfusion rat. Chin J Exp Tradit Med Formulae 21(06):122–126
Chen K, Wu K, Huang D, Huang K, Pang C (2020a) Anti-inflammatory effects of powdered product of Bu Yang Huan Wu decoction: possible role in protecting against transient focal cerebral ischemia. Int J Med Sci 17(12):1854–1863
Chen X, Chen H, He Y, Fu S, Liu H, Wang Q, Shen J (2020b) Proteomics-guided study on Buyang Huanwu decoction for its neuroprotective and neurogenic mechanisms for transient ischemic stroke: involvements of EGFR/PI3K/AKT/Bad/14-3-3 and Jak2/Stat3/cyclin D1 signaling cascades. Mol Neurobiol 57(10):4305–4321
Chen Z, Gong X, Guo Q, Zhao H, Wang L (2019) Bu Yang Huan Wu decoction prevents reperfusion injury following ischemic stroke in rats via inhibition of HIF-1 α, VEGF and promotion β-ENaC expression. J Ethnopharmacol 228:70–81
Cheng YS, Tao ZJ, Wang PL (2022) Effect of Buyang Huanwu decoction on endoplasmic reticulum stress-autophagy in rats with ischemia-reperfusion brain injury. China Modern Doctor 60(11): 34–37+98+197
Cui H, Yang A, Zhou H, Wang C, Luo J, Lin Y, Zong Y, Tang T (2015) Buyang Huanwu decoction promotes angiogenesis via vascular endothelial growth factor receptor-2 activation through the PI3K/Akt pathway in a mouse model of intracerebral hemorrhage. BMC Complement Altern Med 15:91
Ding CJ, Jiang Q, Wang X, Dong LY (2017) Effective fraction from Buyang Huanwu decoction exerts neuroprotective effect on global brain ischemia injury in rodent models via inhibiting lipid peroxidation and apoptosis. Pharmacology and Clinics of Chinese Materia Medica 33(04):2–7
Dou B, Zhou W, Li S, Wang L, Wu X, Li Y, Guan H, Wang C, Zhu S, Ke Z, Huang C, Wang Z (2018) Buyang Huanwu decoction attenuates infiltration of natural killer cells and protects against ischemic brain injury. Cell Physiol Biochem 50(4):1286–1300
Fabian-Jessing BK, Vallentin MF, Secher N, Hansen FB, Dezfulian C, Granfeldt A, Andersen LW (2018) Animal models of cardiac arrest: a systematic review of bias and reporting. Resuscitation 125:16–21
Fukuta T, Asai T, Yanagida Y, Namba M, Koide H, Shimizu K, Oku N (2017) Combination therapy with liposomal neuroprotectants and tissue plasminogen activator for treatment of ischemic stroke. FASEB J 31(5):1879–1890
Gan HY, Li L, Yang Y, Zhuge LJ, Chu LS (2019) Buyang Huanwu decoction inhibits inflammation via regulating microglia/macrophage polarization after cerebral ischemia in rats. Journal of Zhejiang Chinese Medical University 43(1):1–6
Guan L, LIU W, Yan FM, Zhou LQ, Li XY, Xu JW, Liu HM (2015) Effect of Buyang Huanwu decoction on NR2BmRNA expression of cortical neurons after global cerebral ischemia-reperfusion in rats. Lishizhen Medicine and Materia Medica Research 26(06): 1339-1341
Guo JC, Su N, Liu W, Wu SF, Zhou LQ, Guan L, Zhang Y, LI XY (2017) Mechanisms of Buyang Huanwu decoction influencing connexin43 after focal ischemia. Liaoning Journal of Traditional Chinese Medicine 44(11): 2418-2420
Hackam DG, Redelmeier DA (2006) Translation of research evidence from animals to humans. JAMA 296(14):1727
Han C, Kim M, Cho S, Jung W, Moon S, Park J, Ko C, Cho K, Kwon S (2018) Adjunctive herbal medicine treatment for patients with acute ischemic stroke: a systematic review and meta-analysis. Complement Ther Clin Pract 33:124–137
Hao C, Wu F, Shen J, Lu L, Fu D, Liao W, Zheng G (2012) Clinical efficacy and safety of Buyang Huanwu decoction for acute ischemic stroke: a systematic review and meta-analysis of 19 randomized controlled trials. Evidence-Based Complementary and Alternative Medicine 2012:1–10
Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14(1):43
Huang YF, Lou ZH, Deng MJ, Fei YR, Hu WJ (2016) Effect of Buyang Huanwu decoction on the level of inflammatory factors in MCAO model rats. Zhejiang Journal of Traditional Chinese Medicine 51(03):221–222
Jiang C, Xu Y, Zhang W, Pan W, Chao X (2020) Effects and safety of Buyang-Huanwu decoction for the treatment of patients with acute ischemic stroke. Medicine 99(23):e20534
Katan M, Luft A (2018) Global burden of stroke. Semin Neurol 38(02):208–211
Kilkenny C, Browne WJ, Cuthi I, Emerson M, Altman DG (2012) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Vet Clin Pathol 41(1):27–31
Lai RM, Zhu Y, Zheng LX, Zhang JP (2016) Effect of Buyang Huanwu decoction on glycogen synthase kinase 3β mRNA expression in cerebral ischemia-reperfusion injury rat model. J Guangzhou Univ Traditional Chinese Med 33(03):362–366
Li H, Peng D, Zhang S, Zhang Y, Wang Q, Guan L (2021) Buyang Huanwu decoction promotes neurogenesis via sirtuin 1/autophagy pathway in a cerebral ischemia model. Mol Med Rep 24(5):791
Li JH, Liu AJ, Li HQ, Wang Y, Shang HC, Zheng GQ (2014) Buyang Huanwu decoction for healthcare: evidence-based theoretical interpretations of treating different diseases with the same method and target of vascularity. Evid Based Complement Alternat Med 2014:506783
Li ZH (2018) Effects of Buyang Huanwu decoction on platelet aggregation and thrombosis in rats with cerebral ischemia reperfusion. J Chinese Med 33(03):422–425
Liang YX, Zhou GH, Liao ZB, Zhao Q, Zhou RJ, Zhang JP, Qin MN (2019) Effects of Buyang Huanwu decoction on the average opening time of L-type Ca2+ channel in rats with global cerebral ischemia-reperfusion. China Medical Herald 16(16):13–16
Liu W, Guo JC, Su N, Zhang Y, Zhou Y, Zhou LQ (2018) Cx43 mediated protective role of Buyang Huanwu decoction after focal ischemia. Chin Arch Tradit Chin Med 36(9):2068–2070
Liu YL, Wu FH, Yang KL, Zhou Y, Zhou LQ, Liu W (2021) Buyang Huanwu decoction improved cerebral ischemia reperfusion injury in rats by regulating FPR2. Lishizhen Medicine and Materia Medica Research 32(6):1304–1307
Ma J, Xie W (2018) Study on effects of Buyang Huanwu decoction on expression of AKT protein in brain tissue of rats with cerebral ischemia reperfusion injury. Medical Journal of Chinese People’s Health 30(09):1–3
Ma XJ, Zhao YM, Wang WL, Jin XF, Zhou XH, Gao WJ (2022) Buyang Huanwu decoction attenuates cerebral ischemia-reperfusion injury in rats by regulating autophagy through AMPK/mTOR/ULK1 signaling pathway. Chinese Pharmacological Bulletin 38(1):147–152
Ma XQ, Lang FL, Zhao S, Zhang Q (2021) Study on brain protective effect and mechanism of Yiqi Huoxue method based on Wnt/β-catenin signaling pathway on focal cerebral ischemia-reperfusion rats. Guiding J Traditional Chinese Med Pharm 27(11): 30–34+40
McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL (2010) Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol 160(7):1573–1576
Mei Z, Du L, Liu X, Chen X, Tian H, Deng Y, Zhang W (2022) Diosmetin alleviated cerebral ischemia/reperfusion injury in vivo and in vitro by inhibiting oxidative stress via the SIRT1/Nrf2 signaling pathway. Food Funct 13(1):198–212
Mei Z, Huang Y, Feng Z, Luo Y, Yang S, Du L, Jiang K, Liu X, Fu X, Deng Y, Zhou H (2020) Electroacupuncture ameliorates cerebral ischemia/reperfusion injury by suppressing autophagy via the SIRT1-FOXO1 signaling pathway. Aging 12(13):13187–13205
Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. (2020) The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. British Journal of Pharmacology 177(16): 3617–3624
Perel P, Roberts I, Sena E, Wheble P, Briscoe C, Sandercock P, Macleod M, Mignini LE, Jayaram P, Khan KS (2007) Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ 334(7586):197
Qiu M, Zeng WY, Liu W, Ding WT, Yu P (2015) Effects of Buyang Huanwu decoction on connexin43 expression in rats after cerebral ischemia. Journal of Liaoning University of Chinese Medicine 17(05):36–38
Rabinstein AA (2017) Treatment of Acute Ischemic Stroke Continuum (minneap Minn) 23(1):62–81
She Y, Shao L, Zhang Y, Hao Y, Cai Y, Cheng Z, Deng C, Liu X (2019) Neuroprotective effect of glycosides in Buyang Huanwu decoction on pyroptosis following cerebral ischemia-reperfusion injury in rats. J Ethnopharmacol 242:112051
Shen J, Huang K, Zhu Y, Xu K, Zhan R, Pan J (2020) Buyang Huanwu decoction promotes angiogenesis after cerebral ischemia by inhibiting the Nox4/ROS pathway. Evidence-Based Complementary and Alternative Medicine 2020:1–14
Shi WX, Zhou LN (2016) Effect of Buyang Huanwu decoction on hippocampus neurons and 5-HT contents in rats with transient ischemia reperfusion injury. Journal of Liaoning University of Chinese Medicine 18(05):62–64
Tan L, Zhang X, Mei Z, Wang J, Li X, Huang W, Yang S (2016) Fermented Chinese formula Shuan-Tong-Ling protects brain microvascular endothelial cells against oxidative stress injury. Evid Based Complement Alternat Med 2016:5154290
Tu JF, Wei LH, Zhou SA, Zhang K, Chen H, Zhang MQ, Cai WW, Yang Y (2015) Effects of notch conduction system on neural repair of Buyang Huanwu decoction to rats with focal cerebral ischemic-reperfusion injury. Chin Arch Tradit Chin Med 33(03):675–678
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind M, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson U, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW (2020) Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 141(9):e139–e596
Wan HY, Wang HY, He YH, Yang JH, Wan HT, Zhou HF (2021) Protective effect of Buyang Huanwu decoction categorized formula on cerebral ischemia-reperfusion injury in rats. China Journal of Traditional Chinese Medicine and Pharmacy 36(9):5176–5181
Wang H, Liu YL, Zhu M, Wu KN, Zhao MN, Hou HZ, Wei CR, Li YY, Liao XL, Li J, Jia Y, Bian TY, Zhao LL, Ma B (2019) The reporting quality of intervention animal studies published in Chinese journals: a quantitative analysis. Chin J Evid Based Med 19(01):89–96
Wang RS, Cai J, Zhang JP (2015) Effects and mechanisms of Buyang Huanwu decoction on acute cerebral ischemia-reperfusion injury in rats. The Journal of Practical Medicine 31(05):725–727
Wang SX, Yan XL, He YY, Yu AM, Zheng HZ, Wang PC, Zhong NJ, Wen GQ, Wang LS (2020) Laser speckle contrast imaging to observe the effect of Buyang Huanwu decoction on cerebral microvascular flow after cerebral ischemia-reperfusion in rats. Chinese Journal of Hospital Pharmacy 40(19):2007–2012
Wang TF, Fei YR, Pan H, Huang YF (2016) Effects of Buyang Huanwu decoction on TXB-2 and 6-keto-PGF-(1α) in rats with MCAO. Chinese Journal of Hospital Pharmacy 36(10):818–821
Wei C, Wang SL, Kong XG, Zang YY, Li Y (2017) Effects of Buyang Huanwu decoction on the expression of mitochondrial mitotic proteins Drp1, Fis1 and cytochrome C in rats with cerebral ischemia-reperfusion injury. Shanxi Journal of Traditional Chinese Medicine 38(10):1481–1483
Wei R, Teng H, Yin B, Xu Y, Du Y, He F, Chu K, Luo B, Zheng G (2013) A systematic review and meta-analysis of Buyang Huanwu decoction in animal model of focal cerebral ischemia. Evidence-Based Complementary and Alternative Medicine 2013:1–13
Wu FH, Liu YL, Gao YR, Yang KL, Liu W (2021) Buyang Huanwu Tang alleviated oxidative stress following cerebra ischemia/reperfusion in rats by formyl peptide receptor 2. Chin J Exp Tradit Med Formulae 27(18):9–15
Xin ZY, Jin XF, Zhou XH, Liu ZY, Gao P, Ma X, Gao WJ (2022) Buyang-Huanwu decoction reduces cerebral ischemia/reperfusion injury in rats through SIRT1/NF-KB p65 signaling pathway. Chinese Journal of Pathophysiology 38(8):1454–1462
Xu YL, Qin SS, Zhu H, Huang HY, Zheng LX, Zhu Y, Yao H, Zhang JP (2017) Effect of Buyang Huanwu Tang on platelet proteins Src, AKT and p38 MAPK of cerebral ischemia reperfusion injury rat. Chin J Exp Tradit Med Formulae 23(05):135–140
Yang KL, Zhou Y, Yan FM, Zhou LQ, Liu YL, Liu W (2020) Effect of Buyang Huanwu Tang on synaptic structural plasticity after cerebral ischemia-reperfusion in rats. Chin J Exp Tradit Med Formulae 26(01):43–49
Yang T, Chen X, Mei Z, Liu X, Feng Z, Liao J, Deng Y, Ge J (2021) An integrated analysis of network pharmacology and experimental validation to reveal the mechanism of Chinese medicine formula Naotaifang in treating cerebral ischemia-reperfusion injury. Drug Des Devel Ther 15:3783–3808
Ye JB, Shan YD, Tian T, Cai GY, Gao WJ (2022) Effects of Buyang Huanwu decoction on circular RNA expression profile in penumbra of brain tissue in ischemic stroke. China Journal of Traditional Chinese Medicine and Pharmacy 37(5):2525–2530
Zhang Q, Li MZ, Feng XF, Li MC, Zhao H (2021) Effect of Buyang Huanwu decoction on proliferation, differentiation and Wnt/catenin signaling pathway of new neurons in brain area around infarcted area of cerebral ischemia rats. Journal of Beijing University of Traditional Chinese Medicine 44(11):1002–1010
Zhang T, Liao XL, Li B, Bai ZG, Liu YL, Zhao F, Chen HM, Ma B (2019a) A survey of Chinese researchers’ knowledge of animal experimental design methods and reporting standards. Chinese Journal of Evidence-Based Cardiovascular Medicine 11(01):17–23
Zhang WW, Xu F, Wang D, Ye J, Cai SQ (2018) Buyang Huanwu decoction ameliorates ischemic stroke by modulating multiple targets with multiple components: in vitro evidences. Chin J Nat Med 16(3):194–202
Zhang Y, Chen ZY, Liu Q, Song W (2019b) Protective effect of Buyang Huanwu Tang on brain injury in rats following I/R. Chinese Journal of Geriatric Heart Brain and Vessel Diseases 21(08):867–870
Zhang Z, Song J, Jia Y, Zhang Y (2016) Buyanghuanwu decoction promotes angiogenesis after cerebral ischemia/reperfusion injury: mechanisms of brain tissue repair. Neural Regen Res 11(3):435–440
Zhao LD, Wang JH, Jin GR, Zhao Y, Zhang HJ (2012) Neuroprotective effect of Buyang Huanwu decoction against focal cerebral ischemia/reperfusion injury in rats–time window and mechanism. J Ethnopharmacol 140(2):339–344
Zhao Y, Ma X, Yu W, Zhang Z, Wang W, Zhou X, Gao W (2021) Protective effect of Buyang Huanwu decoction on cerebral ischemia reperfusion injury by alleviating autophagy in the ischemic penumbra. Evidence-Based Complementary and Alternative Medicine 2021:1–13
Zheng LX, Zhu Y, Xu YL, Qin SS, Yao H, Zhang JP (2016) Effect of Buyanghuanwu decoction preconditioning on platelet PDK1/AKT Thr308 pathway in acute cerebral ischemia reperfusion rats. Pharmacology and Clinics of Chinese Materia Medica 32(04):1–4
Zheng X, Shan C, Xu Q, Wang Y, Shi Y, Wang Y, Zheng G (2018) Buyang Huanwu decoction targets SIRT1/VEGF pathway to promote angiogenesis after cerebral ischemia/reperfusion injury. Front Neurosci 12:911
Zhou Y, Yang KL, Zhou LQ, Yan FM, Fu WJ, Liu W (2019) Connexin43 mediated neural restoration treatment with Buyang Huanwu decoction after focal ischemia. Chin Arch Tradit Chin Med 37(10):2431–2434
Zhu H, Huang HY, Zhang JP, Yao H, Han B, Li RQ (2015a) Influence of Buyang Huanwu decoction pretreatment on expression of Akt phosphorylation level in cerebral ischemia-reperfusion rats. Traditional Chinese Drug Research and Clinical Pharmacology 26(04):451–455
Zhu XQ, Han J, Hu J, Pang WS (2015b) The protection and mechanism of Buyang Huanwu Tang on focal cerebral ischemia-reperfusion in rats. Chinese Journal of Ethnomedicine and Ethnopharmacy 24(01):42–43
Zhu Y, Cai J, Xu YL, Zhu H, Yao H, Qin SS, Zheng LX, Zhang JP (2015c) Effect of Buyang Huanwu Tang on expression of PTEN mRNA in rat model with cerebral ischemia-reperfusion injury. Chin J Exp Tradit Med Formulae 21(23):135–138
Zhuge LJ, Fang Y, Jin HQ, Li L, Yang Y, Hu XW, Chu LS (2020) Chinese medicine Buyang Huanwu decoction promotes neurogenesis and angiogenesis in ischemic stroke rats by upregulating miR-199a-5p expression. Journal of Zhejiang University (medical Sciences) 49(06):687–696
Funding
This work was supported by the fund of the National Natural Science Foundation of China (82174167), the project of Natural Science Foundation of Hunan Province (2021JJ30499), the fund for Youth Top Talent Project of Hubei Provincial Health and Family Planning Commission (EWT-2019-48), and Key Projects of Hunan Province Education Department (20A366).
Author information
Authors and Affiliations
Contributions
ZGM and JWG conceived and designed the study. XYC, TY, and ZGM selected, extracted, and appraised the data. XYC drafted the manuscript. YNL, ZTF, and RF provided some positive suggestions and amended the manuscript. All authors read and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent for publication
All authors have signed the consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, X., Yang, T., Luo, Y. et al. Methodological and reporting quality evaluation of Buyang Huanwu decoction for experimental cerebral ischemia–reperfusion injury: a systematic review. Naunyn-Schmiedeberg's Arch Pharmacol 396, 831–849 (2023). https://doi.org/10.1007/s00210-022-02362-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02362-9