Abstract
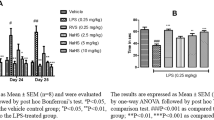
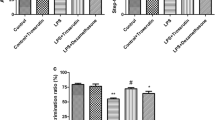
d-Ribose-l-cysteine (DRLC), an analog of cysteine that boosts glutathione (GSH) content, has been reported to mitigate oxidative stress–mediated diseases. This study seeks to evaluate the effects of DRLC on memory deficits and the biochemical and histo-morphological changes induced by lipopolysaccharide (LPS) in mice. Male Swiss mice (n = 10) were pre-treated orally with three doses of DRLC (25 mg/kg, 50 mg/kg, and 100 mg/kg), donepezil (1 mg/kg), or vehicle (saline) for 30 min prior to the intraperitoneal injection of LPS (0.25 mg/kg) daily for 7 days. Memory functions were evaluated using the Y-maze, object recognition, and social recognition tests. The specific brain regions (prefrontal cortex and hippocampus) were evaluated to determine oxidative stress biomarkers (malondialdehyde, GSH, and catalase), acetyl-cholinesterase activity, proinflammatory cytokines (tumor necrosis factor-α and interleukin-6), expression of nuclear factor-kappa B (NF-κB), and neuronal cell morphology. DRLC (25–100 mg/kg) reversed the memory deficits in the LPS-treated mice (p < 0.05). The increased oxidative stress and proinflammatory cytokines in the brain regions of the LPS-treated mice were significantly (p < 0.05) reduced by DRLC. DRLC (50 mg/kg and 100 mg/kg) also reduced acetyl-cholinesterase activity and decreased NF-κB expression in the brains of LPS-treated mice. Finally, it attenuated the cytoarchitectural distortions and loss of neuronal cells of the prefrontal cortex and hippocampus that were induced by LPS in mice. The results of this study suggest that DRLC attenuates memory deficit induced by LPS in mice through mechanisms related to the inhibition of oxidative stress, release of proinflammatory cytokines, and expression of NF-κB in mice.














Similar content being viewed by others
References
Adair JC, Knoefel JE, Morgan N (2001) Controlled trial of N-acetylcysteine for patients with probable Alzheimer’s disease. Neurology 57:1515–1517
Adam-Vizi V, Seregi A (1982) Receptor independent stimulatory effect of noradrenaline on Na+/K+-ATPase in rat brain homogenate, role of lipid peroxidation. Biochem Pharmacol 34:2231–2236
Adolphs R (1982) The neurobiology of social cognition. Curr Opin Neurobiol 11:231–239
Agostinho P, Cunha RA, Oliveira C (2010) Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr Pharm Des 16:2766–2778
Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA (2006) Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci 9:119–126
Ben-Azu B, Aderibigbe AO, Ajayi AM, Eneni AO, Omogbiya IA, Owoeye O, Umukoro S, Iwalewa EO (2019) Morin decreases cortical pyramidal neuron degeneration via inhibition of neuroinflammation in mouse model of schizophrenia. Int Immunopharmacol 70:338–345
Block ML, Hong JS (2005) Microglia and inflammation-mediated neurodegeneration. Multiple triggers with a common mechanism. Prog Neurobiol 76:77–98
Blokland A (2005) Scopolamine-induced deficits in cognitive performance: a review of animal studies. Scopolamine Rev 1:1–76
Bluthé RM, Pawlowski M, Suarez S, Parnet P, Pittman Q, Kelley KW, Dantzer R (1994a) Synergy between tumor necrosis factor α and interleukin-1 in the induction of sickness behaviour in mice. Psychoneuroendocrinol 19:197–207
Bluthé RM, Walter V, Parnet P, Layé S, Lestage J, Verrier D, Poole S, Stenning BE, Kelley KW, Dantzer R (1994b) Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci III 317:499–503
Borgström L, Kågedal B, Paulsen O (1986) Pharmacokinetics of N-acetylcysteine in man. Eur J Clin Pharmacol 31:217–222
Burns A, Jacoby R, Levy R (1990) Psychiatric phenomena in Alzheimer's disease I: disorders of thought content. Br J Psychiatry J Ment Sci 157:72–76
Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA (2006) Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochim Biophys Acta 1762:447–452
Contestabile A (2011) The history of the cholinergic hypothesis. Behav Brain Res 221:334–340
Czerniawski J, Miyashita T, Lewandowski G, Guzowski JF (2015) Systemic lipopolysaccharide administration impairs retrieval of context-object discrimination, but not spatial, memory: evidence for selective disruption of specific hippocampus dependent memory functions during acute neuroinflammation. Brain Behav Immun 44:159–166
Dluzen DE, Kreutzberg JD (1993) 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) disrupts social memory/recognition processes in the male mouse. Brain Res 609:98–102
Ek M, Kurosawa M, Lundeberg T, Ericsson A (1998) Activation of vagal afferents after intravenous injection ofinterleukin-1beta: role of endogenous prostaglandins. J Neurosci 18:9471–9479
Ellman GL, Courtney KD, Andre JV, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Frühauf PKS, Ineu RP, Tomazi L, Duarte T, Mello CF, Rubin MA (2015) Spermine reverses lipopolysaccharide-induced memory deficit in mice. J Neuroinflammation 12:1–11
Gao J, Xiong B, Zhang B, Li S, Huang N, Zhan G, Jiang R, Yang L, Wu Y, Miao L, Zhu B, YangC LA (2018) Sulforaphane alleviates lipopolysaccharide-induced spatial learning and memory dysfunction in mice: the role of BDNF-mTOR signaling pathway. Neuroscience 388:357–366
Ghosh S, Lertwattanarak R, Garduño JDE, Galeana JJ, Li J, Zamarripa F, Lancaster JL, Mohan S, Hussey S, Musi N (2015) Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. J Gerontol A Biol Sci Med Sci 70:232–246
Goehler LE, Gaykema RP, Nguyen KT, Lee JE, Tilders FJ, Maier SF, Watkins LR (1999) Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci 19:2799–2806
Gonçalves C, Dos Santos DB, Portilho SS, Lopes MW, Ghizoni H, de Souza V, Mack JM, Naime AA, Dafre AL, de Souza BP, Prediger RD, Farina M (2018) Lipopolysaccharide-induced striatal nitrosative stress and impaired social recognition memory are not magnified by paraquatco exposure. Neurochem Res 43:745–759
Green LC, Tannenbaum SR, Goldman P (1981) Nitrate synthesis in the germ free and conventional rat. Science 212:56–58
Hauss-Wegrzyniak B, Vannucchi MG, Wenk GL (2000) Behavioral and ultrastructural changes induced by chronic neuroinflammation in young rats. Brain Res 859:157–166
Houdek HM, Larson J, Watt JA, Rosenberger TA (2014) Bacterial lipopolysaccharide induces a dose-dependent activation of neuroglia and loss of basal forebrain cholinergic cells in the rat brain. Inflamm Cell Signal 1:e47
Jalkanen J, Puttonen KA, Venalainen JI, Sinervä V, Mannila A, Ruotsalainen S, Jarho EM, Wallén EA, Männistö PT (2007) Beneficial effect of prolyloligopeptidase inhibition on spatial memory in young but not in old scopolamine-treated rats. Basic Clin Pharmacol Toxicol 100:132–138
Jang JH, Surh YJ (2005) Beta-amyloid-induced apoptosis is associated with cyclooxygenase-2 upregulation via the mitogen-activated protein kinase-NF-kappaB signaling pathway. Free Radic Biol Med 38:1604–1613
Kader T, Porteous CM, Williams MJ, Gieseg SP, McCormick SP (2014) Ribose-cysteine increases glutathione-based antioxidant status and reduces LDL in human lipoprotein(a) mice. Atherosclerosis 237:725–733
Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C, Tyagi N (2016) Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer's disease: understanding the therapeutics strategies. Mol Neurobiol 53:648–661
Kanter MZ (2006) Comparison of oral and i.v. acetylcysteine in the treatment of acetaminophen poisoning. Am J Health Syst Pharm 63:1821–1827
Kaplowitz N, Aw TY, Ookhtens M (1985) The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol 25:715–744
Lai WS, Ramiro LL, Yu HA, Johnston RE (2005) Recognition of familiar individuals in Golden hamsters: a new method and functional neuroanatomy. J Neurosci 25:11239–11247
Lowes DA, Webster NR, Murphy MP, Galley HF (2013) Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. B J Anaesth 110:472–480
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta 1830:3143–3153
Lucas AM, Hennig G, Domnick PK, Whiteley HE, Roberts JC, Cohen SD (2000) Ribose cysteine protects against acetaminophen-induced hepatic and renal toxicity. Toxicol Pathol 28:697–704
Mandal PK, Saharan S, Tripathi M, Murari G (2015) Brain glutathione levels-a novel biomarker for mild cognitive impairment and Alzheimer’s disease. Biol Psychiatry 78:702–710
Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med 23:134–147
Mazzanti G, Di Giacomo S (2016) Curcumin and resveratrol in the management of cognitive disorders: what is the clinical evidence? Molecules 21:9
Ming Z, Wotton CA, Appleton RT, Ching JC, Loewen ME, Sawicki G, Bekar LK (2015) Systemic lipopolysaccharide-mediated alteration of cortical neuromodulation involves increases in monoamine oxidase-a and acetylcholinesterase activity. J Neuroinflammation 12:37–47
Moreira PI, Santos MS, Oliveira CR, Shenk JC, Nunomura A, Smith MA, Zhu X, Perry G (2008) Alzheimer disease and the role of free radicals in the pathogenesis of thedisease. CNS Neurol Disord Drug Targets 7:3–10
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. GLIA 55:453e462
Roberts JC, Nagasawa HT, Zera RT, Fricke RF, Goon DJ (1987) Prodrugs of L-cysteine as protective agents against acetaminophen-induced hepatotoxicity. J Med Chem 30:1891–1896
Roberts JC, Francetic DJ (1991) Time course for the elevation of glutathione in numerous organs of L1210-bearing CDF1 mice given the L-cysteine prodrug, RibCys. Toxicol Lett 59:245–251
Roberts JC, Charyulu RL, Zera RT, Nagasawa HT (1992) Protection against acetaminophen hepatotoxicity by ribose-cysteine (Rib-Cys). Pharmacol Toxicol 70:281–285
Sawda C, Moussa C, Turner RS (2017) Resveratrol for Alzheimer’s disease. An NY Acad Sci 1403:142–149
Shimazaki T, Kaku A, Chaki S (2010) D-serine and a glycine transporter-1 inhibitor enhance social memory in rats. Psychopharmacology (Berlin) 209:263–270
Silva LI, Carter SD, Cooper CV, Aparachita P, Shili C, Usry JL, Perryman KR (2018) Effects of oral administration of lipopolysaccharide on growth performance and immune response of nursery pigs. J Anim Sci 96:209
Sinha KA (1971) Colorimetric assay of catalase. Anal Biochem 47:389–394
Sun J, Zhang S, Zhang X, Zhang X, Dong H, Qian Y (2015) IL-17A is implicated in lipopolysaccharide-induced neuroinflammation and cognitive impairment in aged rats via microglial activation. J Neuroinflammation 12:165
Taglialatela G, Hogan D, Zhang WR, Dineley KT (2009) Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav Brain Res 200:95–99
Terry AW, Buccafusco JJ (2003) The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther 306:821–827
Thor DH, Holloway WR (1982) Social memory of the male laboratory rat. J Comp Physiol Psychol 96:1000–1006
Tiwari SC, Soni RM (2014) Alzheimer’s disease pathology and oxidative stress: possible therapeutic options. J Alzheimers Dis 4:162
Tonnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis 57:1105–1121
Tyagi E, Agrawal R, Nath C, Shukla R (2010) Effect of melatonin on neuroinflammation and acetylcholinesterase activity induced by LPS in rat brain. Eur J Pharmacol 640:206–210
Vale JA, Proudfoot AT (1995) Paracetamol (acetaminophen) poisoning. Lancet 346:547–552
Wan W, Wetmore L, Sorensen CM, Greenberg AH, Nance DM (1994) Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res Bull 34:7–14
Wang W, Li S, Dong H, Lv S, Tang Y (2009) Differential impairment of spatial and nonspatial cognition in a mouse model of brain aging. Life Sci 85:127–135
Weintraub MK, Bissona CM, Nouria JN, Vinsona BT, Eimerbrink MJ, Kranjac D, Boehm GW, Chumley MJ (2013) Imatinibmethanesulfonate reduces hippocampal amyloid-beta and restores cognitive function following repeated endotoxin exposure. Brain Behav Immun 33:24–28
Wojsiat J, Zoltowska KM, Laskowska-Kaszub K, Wojda U (2018) Oxidant/antioxidant imbalance in Alzheimer’s disease: therapeutic and diagnostic prospects. Oxidative Med Cell Longev 2018:6435861 1-16
Zhu F, Zheng Y, Ding Y, Liu Y, Zhang X, Wu R, Xiaofeng G, Jingping Z (2014) Minocycline and risperidone prevent microglia activation and rescue behavioural deficits induced by neonatal intrahippocampal injection of lipopolysaccharide in rats. PLoS One 9:e93966
Acknowledgments
We thank the technical staff of the Department of Pharmacology and Therapeutics, University of Ibadan, Ibadan for their kind assistance. We would also like to acknowledge Michael O. S. Afolabi, PhD, Postdoctoral Fellow, Department of Pediatrics and Child Health, University of Manitoba, Canada, for his editorial assistance with the manuscript.
Author information
Authors and Affiliations
Contributions
OE, BB, and SU conceived and designed the study. OE, BB, and AMA conducted experiments. OE and BB analyzed data. BB and SU wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Emokpae, O., Ben-Azu, B., Ajayi, A.M. et al. d-Ribose-l-cysteine attenuates lipopolysaccharide-induced memory deficits through inhibition of oxidative stress, release of proinflammatory cytokines, and nuclear factor-kappa B expression in mice. Naunyn-Schmiedeberg's Arch Pharmacol 393, 909–925 (2020). https://doi.org/10.1007/s00210-019-01805-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01805-0