Abstract
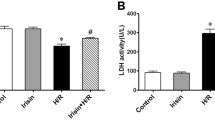
Endoplasmic reticulum (ER) stress–induced apoptosis is a major cause of myocardial ischemia/reperfusion (I/R) injury. Emerging evidence indicates that glucagon-like peptide-1 (GLP-1) has potential cardioprotective effects. However, the precise mechanisms underlying the involvement of GLP-1 in I/R injury remain largely unknown. In the present study, we aimed to determine whether GLP-1 attenuates hypoxia/reoxygenation (H/R) injury in cardiomyocytes and to further elucidate the underlying signaling pathway. The results indicate that GLP-1 reversed the increased apoptotic ratio, the increased lactate dehydrogenase (LDH) levels, the reduced cell viability, the increased Caspase-3 activity, and the increased Bax/Bcl-2 ratio caused by H/R. Importantly, GLP-1 significantly decreased the expression of H/R-induced ER stress proteins (GRP78, CHOP) and Caspase-12. In addition, we found that GLP-1 increased the expression of p-Akt in H9c2 cells with H/R injuries, and that the protective action of GLP-1 against H/R-induced injury was blocked by the GLP-1 receptor (GLP-1R) inhibitor Exendin9-39 and the PI3K inhibitor LY294002. Exendin9-39 and LY294002 also blocked the downregulation of ER stress protein expression by GLP-1, after H/R injury. Therefore, we have shown that GLP-1 exerts its cardioprotective effects by alleviating ER stress–induced apoptosis due to H/R injury and that these effects are most likely associated with the activation of GLP-1R/PI3K/Akt signaling pathway.





Similar content being viewed by others
References
Basalay MV, Mastitskaya S, Mrochek A, Ackland GL, del Arroyo AG, Sanchez J, Sjoquist P-O, Pernow J, Gourine AV, Gourine A (2016) Glucagon-like peptide-1 (GLP-1) mediates cardioprotection by remote ischaemic conditioning. Cardiovasc Res 112:669–676
Birnbaum Y, Ye Y, Bajaj M (2012) Myocardial protection against ischemia-reperfusion injury by GLP-1: molecular mechanisms. Metab Syndr Relat Disord 10:387–390
Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM (2005) Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes 54:146–151
Chang G, Zhang D, Yu H, Zhang P, Wang Y, Zheng A, Qin S (2013) Cardioprotective effects of exenatide against oxidative stress-induced injury. Int J Mol Med 32:1011–1020
Chang G, Liu J, Qin S, Jiang Y, Zhang P, Yu H, Lu K, Zhang N, Cao L, Wang Y (2017) Cardioprotection by exenatide: a novel mechanism via improving mitochondrial function involving the GLP-1 receptor/cAMP/PKA pathway. Int J Mol Med 41
Chen F, Li Q, Zhang Z, Lin P, Lei L, Wang A, Jin Y (2015) Endoplasmic reticulum stress cooperates in zearalenone-induced cell death of RAW 264.7 macrophages. Int J Mol Sci 16:19780–19795
Chinda K, Chattipakorn S, Chattipakorn N (2012) Cardioprotective effects of incretin during ischaemia-reperfusion. Diab Vasc Dis Res 9:256–269
Cho YM, Fujita Y, Kieffer TJ (2014) Glucagon-like peptide-1: glucose homeostasis and beyond. Annu Rev Physiol 76:535–559
Doroudgar S, Glembotski CC (2013) New concepts of endoplasmic reticulum function in the heart: programmed to conserve. J Mol Cell Cardiol 55:85–91
González N, Acitores A, Sancho V, Valverde I, Villanueva-Peñacarrillo ML (2005) Effect of GLP-1 on glucose transport and its cell signalling in human myocytes. Regul Pept 126:203–211
Guo J, Bian Y, Bai R, Li H, Fu M, Xiao C (2013) Globular adiponectin attenuates myocardial ischemia/reperfusion injury by upregulating endoplasmic reticulum Ca2+-ATPase activity and inhibiting endoplasmic reticulum stress. J Cardiovasc Pharmacol 62:143–153
Heo KS, Fujiwara K, Abe J (2012) Glucagon-like peptide-1 and its cardiovascular effects. Curr Atheroscler Rep 14:422–428
Huang M, Wei R, Wang Y, Su T, Li Q, Yang X, Chen X (2017) Protective effect of glucagon-like peptide-1 agents on reperfusion injury for acute myocardial infarction: a meta-analysis of randomized controlled trials. Ann Med 49:552–561
Jennings RB (2013) Historical perspective on the pathology of myocardial ischemia/reperfusion injury. Circ Res 113:428–438
Lei Y, Guan G, Lei L, Lv Q, Liu S, Zhan X, Jiang Z, Xiang G (2018) Palmitic acid induces human osteoblast-like Saos-2 cell apoptosis via endoplasmic reticulum stress and autophagy. Cell Stress Chaperon: 1–12
Lønborg J, Vejlstrup N, Kelbæk H, Bøtker HE, Kim WY, Mathiasen AB, Jørgensen E, Helqvist S, Saunamäki K, Clemmensen P (2011) Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J 33:1491–1499
Mccullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21:1249–1259
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner ABA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103
Neuber C, Uebeler J, Schulze T, Sotoud H, Elarmouche A, Eschenhagen T (2014) Guanabenz interferes with ER stress and exerts protective effects in cardiac myocytes. PLoS One 9:e98893
Roos ST, Timmers L, Biesbroek PS, Nijveldt R, Kamp O, van Rossum AC, van Hout GP, Stella PR, Doevendans PA, Knaapen P (2016) No benefit of additional treatment with exenatide in patients with an acute myocardial infarction. Int J Cardiol 220:809–814
Rowlands J, Carlessi R, Newsholme P, Heng J (2018) Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism and function. Front Endocrinol 9:672
Saraiva FK, Sposito AC (2014) Cardiovascular effects of glucagon-like peptide 1 (GLP-1) receptor agonists. Cardiovasc Diabetol 13:142
Schisano B, Harte AL, Lois K, Saravanan P, Aldaghri N, Alattas O, Knudsen LB, Mcternan PG, Ceriello A, Tripathi G (2012) GLP-1 analogue, Liraglutide protects human umbilical vein endothelial cells against high glucose induced endoplasmic reticulum stress. Regul Pept 174:46–52
Shi L, Ji Y, Jiang X, Zhou L, Xu Y, Li Y, Jiang W, Meng P, Liu X (2015) Liraglutide attenuates high glucose-induced abnormal cell migration, proliferation, and apoptosis of vascular smooth muscle cells by activating the GLP-1 receptor, and inhibiting ERK1/2 and PI3K/Akt signaling pathways. Cardiovasc Diabetol 14:18
Singh N, Dhalla AK, Seneviratne C, Singal PK (1995) Oxidative stress and heart failure. Mol Cell Biochem 147:77–81
Sun MY, Ma DS, Zhao S, Wang L, Ma CY, Bai Y (2018) Salidroside mitigates hypoxia/reoxygenation injury by alleviating endoplasmic reticulum stress-induced apoptosis in H9c2 cardiomyocytes. Mol Med Rep
Szegezdi E, Fitzgerald U, Samali A (2003) Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci 1010:186–194
Vilatoba M, Eckstein C, Bilbao G, Smyth CA, Jenkins S, Thompson JA, Eckhoff DE, Contreras JL (2005) Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum-stress mediated apoptosis. Surgery 138:342–351
Wei Y, Mojsov S (1995) Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett 358:219–224
Wei Q, Bin W, Yan B, Xiaohong Z, Jipeng Y, Miaomiao X, Yue G, Qing S, Yichen C, Qiangju W (2014) Magnesium lithospermate B improves myocardial function and prevents simulated ischemia/reperfusion injury-induced H9c2 cardiomyocytes apoptosis through Akt-dependent pathway. J Ethnopharmacol 151:714–721
Wu N, Zhang X, Jia P, Jia D (2015) Hypercholesterolemia aggravates myocardial ischemia reperfusion injury via activating endoplasmic reticulum stress-mediated apoptosis. Exp Mol Pathol 99:449–454
Xie Z, Enkhjargal B, Wu L, Zhou K, Sun C, Hu X, Gospodarev V, Tang J, You C, Zhang JH (2018) Exendin-4 attenuates neuronal death via GLP-1R/PI3K/Akt pathway in early brain injury after subarachnoid hemorrhage in rats. Neuropharmacology 128:142–151
Xu J, Hu H, Chen B, Yue R, Zhou Z, Liu Y, Zhang S, Xu L, Wang H, Yu Z (2015) Lycopene protects against hypoxia/reoxygenation injury by alleviating ER stress induced apoptosis in neonatal mouse cardiomyocytes. PLoS One 10:e0136443
Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, Pipeleers D, Ling Z, Drucker DJ (2006) GLP-1 receptor activation improves β cell function and survival following induction of endoplasmic reticulum stress. Cell Metab 4:391–406
Zhang Y, Zhou H, Wu W, Shi C, Hu S, Yin T, Ma Q, Han T, Zhang Y, Tian F (2016) Liraglutide protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury through the suppression of the SR-Ca(2+)-XO-ROS axis via activation of the GLP-1R/PI3K/Akt/survivin pathways. Free Radic Biol Med 95:278–292
Zhu X, Zhang ZL, Li P, Liang WY, Feng XR, Liu ML (2013) GW24-e1859 Shenyuan, an extract of American ginseng and corydalis tuber formula, attenuates cardiomyocyte apoptosis via inhibition of endoplasmic reticulum stress and oxidative stress in a porcine model of myocardial infarction. J Ethnopharmacol 150:672–681
Acknowledgments
We thank professor Lei Yang (Basic Medical Science College, Jiujiang University, Jiujiang, Jiangxi Province, China) for providing intellectual content of critical importance to the work described.
Funding
This research study was funded by the National Natural Science Foundation of China (Grant No. 81660152) and the Natural Science Foundation of Jiangxi Province (Grant No.20181BAB205004).
Author information
Authors and Affiliations
Contributions
X.G. designed the study. G.G., J.Z., and S.L. performed the experiments and collected the data. W.H. and Y.G. analyzed and interpreted the experimental data. X.G. and G.G. prepared the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. S1
Determination of the cytotoxicity of H/R to H9c2 cells over various time intervals. The cells were treated with different durations of hypoxia, followed by reoxygenation for 6 h, and then processed for the CCK8 assay. Statistical analysis is shown on the bar graphs. Data are presented as the mean ± SEM of three independent experiments. *P < 0.05 versus control. (PNG 13 kb)
Supplementary Fig. S2
GLP-1R is expressed in H9c2 cells. GLP-1R expression was measured by western blot analysis in normal H9c2 cells. (PNG 26 kb)
Supplementary Fig. S3
Exendin9–39 abolished the effects of GLP-1 on cAMP levels, phosphorylation of CREB, and phosphorylation of ERK1/2 in H/R-treated H9c2 cells. H9c2 cells were treated with Exendin9–39 before GLP-1 treatment in H/R-injury and then processed for the cAMP levels assay (A). Western blot analysis of p-CREB, CREB, p-ERK1/2, and ERK1/2 in H/R-injured H9c2 cells treated with Exendin9–39 before GLP-1 treatment (B). The analysis of band intensities is presented as the relative ratio of p-CREB to CREB and the relative ratio of p-ERK1/2 to ERK1/2 (C). G: GLP-1; E: Exendin9-39. Statistical analysis is shown on the bar graphs. Data are presented as the mean ± SEM of three independent experiments. *P < 0.05 versus control, #P < 0.05 versus H/R, &P < 0.05 versus H/R+GLP-1. (PNG 126 kb)
Supplementary Table S1
Antibody details. (DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Guan, G., Zhang, J., Liu, S. et al. Glucagon-like peptide-1 attenuates endoplasmic reticulum stress–induced apoptosis in H9c2 cardiomyocytes during hypoxia/reoxygenation through the GLP-1R/PI3K/Akt pathways. Naunyn-Schmiedeberg's Arch Pharmacol 392, 715–722 (2019). https://doi.org/10.1007/s00210-019-01625-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01625-2