Abstract
Cough is currently the most common reason for patients to visit a primary care physician in the UK, yet it remains an unmet medical need. Current therapies have limited efficacy or have potentially dangerous side effects. Under normal circumstances, cough is a protective reflex to clear the lungs of harmful particles; however, in disease, cough can become excessive, dramatically impacting patients’ lives. In many cases, this condition is linked to inflammatory diseases such as asthma and chronic obstructive pulmonary disease (COPD), but can also be refractory to treatment and idiopathic in nature. Therefore, there is an urgent need to develop therapies, and targeting the sensory afferent arm of the reflex which initiates the cough reflex may uncover novel therapeutic targets. The cough reflex is initiated following activation of ion channels present on vagal sensory afferents. These ion channels include the transient receptor potential (TRP) family of cation-selective ion channels which act as cellular sensors and respond to changes in the external environment. Many direct activators of TRP channels, including arachidonic acid derivatives, a lowered airway pH, changes in temperature, and altered airway osmolarity are present in the diseased airway where responses to challenge agents which activate airway sensory nerve activity are known to be enhanced. Furthermore, the expression of some TRP channels is increased in airway disease. Together, this makes them promising targets for the treatment of chronic cough. This review will cover the current understanding of the role of the TRP family of ion channels in the activation of airway sensory nerves and cough, focusing on four members, transient receptor potential vanilloid (TRPV) 1, transient receptor potential ankyrin (TRPA) 1, TRPV4, and transient receptor potential melastatin (TRPM) 8 as these represent the channels where most information has been gathered with relevance to the airways. We will describe recent data and highlight the possible therapeutic utility of specific TRP channel antagonists as antitussives in the clinic.
Similar content being viewed by others
Introduction
Cough is a troublesome symptom and is currently the most common reason for visiting a doctor in the UK (Schappert and Burt 2006; Schappert and Rechtsteiner 2011). Epidemiological studies have shown that at any one time, up to 40 % of the population reports cough (Janson et al. 2001). Cough is normally an important defensive reflex, which protects the airways from the inhalation of harmful substances and foreign materials and aids in immune defence (Widdicombe 1995; Fontana et al. 1999; Irwin et al. 1998); indeed when cough is ineffective, this can lead to a variety of pathological conditions including atelectasis, bronchiectasis, pneumonia, lung abscesses, and pulmonary scarring (Madison and Irwin 2010). However, in certain diseases, the cough response can become augmented, leading to excessive coughing which can be due to increased activation of the neuronal pathways responsible (Young and Smith 2011).
Clinically, the aetiology and treatment of cough can broadly be classified into either acute (less than 3 weeks) (Irwin et al. 1998), which is largely as a result of viral upper respiratory tract infections (URTIs) and bacterial infections (Curley et al. 1988; Irwin et al. 1998), and chronic cough (more than 8 weeks in duration) which can lead to an increase in both frequency and intensity of cough (McGarvey et al. 2007). Chronic cough has been estimated to affect up to 40 % of the population at any one time (Cullinan 1992; Janson et al. 2001; Morice et al. 2001). Chronic cough is often associated with inflammatory airway diseases including chronic obstructive pulmonary disease (COPD), asthma, pulmonary fibrosis, lung cancer, or conditions outside the lung such as gastro-oesophageal reflux and rhinosinusitis (Fuller and Choudry 1987; Irwin et al. 1998; Morice et al. 2007a) and is also a side effect of drug treatments such as ACE inhibitors (Sesoko and Kaneko 1985; Lalloo et al. 1996; Fahim et al. 2011; Faruqi et al. 2014). Chronic cough can also be idiopathic in origin, and this can account for 18–42 % of patients at specialist cough clinics (Polley et al. 2008; Haque et al. 2005), and chronic cough severe enough to interfere with the normal activities of daily life is thought to affect approximately 7 % of the population (Ford et al. 2006). Chronic cough patients report cough on exposure to a broad range of normally innocuous stimuli including environmental, mechanical, and temperature changes (Birrell et al. 2009; Khalid et al. 2014). This is a debilitating condition, where long-term excessive cough has side effects including sleep disturbance, nausea, chest pains, social embarrassment, incontinence and depression, alongside affecting social relationships with family and colleagues (Brignall et al. 2008). This condition can affect patients, for months or years at a time, ultimately impacting on the quality of life for sufferers (French 1998).
Antitussive medications represent one of the most widely sold over-the-counter (OTC) medications, and in the USA alone, over $3 billion a year is spent on antitussive therapies (Footitt and Johnston 2009) and £100 million in the UK (Dicpinigaitis et al. 2011). A survey carried out in the USA of over 8000 subjects reported that over half of preschool children had been given OTCs, of which 67 % were composed of cough medications (Kogan et al. 1994). This is despite the fact that a systematic review including 2100 participants, found a lack of evidence to support the use of OTC cough therapies (Schroeder and Fahey 2002). Many OTC medications are thought to have no pharmacological effects and instead act as a lubricant to coat the throat (Eccles 2006). The American College of Chest Physicians (ACCP) now advises against OTC therapy for cough with URTI (Bolser 2006), and furthermore, some children’s cough and cold medications in the USA have now been removed from public use due to potentially life-threatening side effects in children under 2 years, in the absence of evidence of efficacy (Centers for Disease Control and Prevention 2007; Vassilev et al. 2009).
Treatments can be broadly classified into those that act centrally, such as opiates, and those which act peripherally, outside the central nervous system targeting airway sensory nerves. Opiates, which exert their actions through the central nervous system, are the only class of drug currently in use that have shown any efficacy and are therefore the current gold standard in cough treatment. A clinical trial demonstrated that morphine sulphate was efficacious in treating chronic cough in patients that did not respond to specific antitussive treatments (Morice et al. 2007b). Clinically, codeine is currently the reference antitussive of choice, but doses used have been shown to have side effects such as dependence, respiratory depression, and GI problems (Belvisi and Geppetti 2004). In addition, a clinical trial has shown that treatment with codeine in patients with COPD and cough was no more effective than placebo (Smith et al. 2006a).
Despite the high prevalence, treatment of chronic cough is a significant unmet medical need, and there is an urgent requirement for new, safe and effective therapies with fewer side effects. Currently, most therapies are targeted at treating the underlying cause of cough (if they can be identified), but in many cases, these are not effective; therefore, treatments specifically targeting cough are needed. Patients with asthma, COPD, post viral cough and interstitial lung diseases exhibit increased cough reflex sensitivity to inhaled tussive agents, suggesting that targeting sensory nerve activation and the peripheral arm of the reflex response may be effective in treating the excessive cough (Key et al. 2010). Members of the transient receptor potential (TRP) family of ion channels are present on vagal sensory nerves, which, when activated, initiate the cough reflex. TRP channels are a family of cation-selective ion channels which act as cellular sensors and respond to changes in the external environment. Many direct activators including arachidonic acid derivatives, a lowered airway pH, changes in temperature, reactive oxygen species (ROS), oxidative stress by-products and altered airway osmolarity are present in the diseased airway of patients that exhibit increased symptoms such as cough. Furthermore, some TRP channels have shown altered or increased expression in airway disease. Together, this makes them promising targets for the treatment of chronic cough.
The aims of this article are to review the current understanding of airway sensory nerves and the cough reflex and to highlight the role of the TRP family of ion channels present on vagal nerve afferents as potential therapeutic targets.
Physiology of the cough reflex
The cough reflex is a forced event, which clears the larynx, trachea and large bronchi of secretions. There are three phases, which begin with a rapid deep inspiration, followed by a compressive phase where the glottis closes and there is a build-up of intrathoracic pressure. The cough ends with an expiratory phase where the glottis opens and there is a rapid expulsion of air and characteristic cough sound, which clears the airways of irritant material. During this final phase, air expulsion speed can reach 500 mph (Bianco et al. 1988). Coughs can occur in bouts where a number of repetitive coughs occur in succession (Nasra and Belvisi 2009). Although the cough reflex is involuntary, cough can be induced and mimicked voluntarily.
Cough and airway sensory nerves
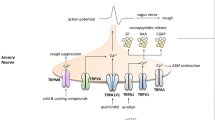
Regardless of cause, the cough reflex is generally initiated following activation of airway sensory nerves. Studies carried out in animals have determined that the cough reflex is regulated by vagal afferent nerves (Canning et al. 2006), which innervate the airways along with other midline organs and tissues (Standring 2005). Receptors present on the vagal nerve termini situated in and under the airway epithelium can be activated by a wide variety of stimuli. These include mechanical stimuli such as punctate touch and lung inflation (Canning 2004), inflammatory mediators released during disease such as PGE2 and bradykinin (Kawakami et al. 1973; Choudry et al. 1989; Maher et al. 2009; Grace et al. 2012), environmental irritants such as cigarette smoke and pollutants (Lee et al. 2007; Belvisi et al. 2011), changes in osmolarity of the airways (Lowry et al. 1988; Koskela et al. 2005) and changes in pH or temperature (McGarvey et al. 1998; Wong et al. 1999). Activation of these receptors causes a localized membrane depolarization, and if this reaches a certain threshold, then, an action potential is generated following activation of voltage-gated sodium channels (VGSCs) and carried up by the vagus nerve to the brainstem. The nerve terminals then synapse in the nucleus tract solitarius (nTS), where the reflex is processed, and finally, the information is carried by efferent motor neurons to the respiratory muscles and larynx to cause cough (Fig. 1). Initiation of cough requires a sustained high frequency activation of the afferent nerves that creates an urge to cough in patients preceding the reflex itself (Canning and Mori 2011; Dicpinigaitis et al. 2014)
The cough reflex. The cough reflex can be separated into three components, the peripheral arm which initiates the reflex following sensory nerve activation, a central component where the reflex is processed in the brainstem and, finally, the efferent arm which causes the cough response following activation of motor nerves. The cough reflex initiates when endogenous or exogenous irritants activate receptors present on sensory nerves in the airways. If this activation reaches a certain threshold, then an action potential is produced, which is carried up by the vagus nerve which synapses in the nucleus tractus solitarius (nTS) in the brainstem. A signal is then sent down to motor neurons to the diaphragm, respiratory muscles and larynx to cause the characteristic cough response
The cell bodies for airway sensory nerves are mostly housed in the nodose and jugular ganglia although around 1 % originate from the thoracic dorsal root ganglia (DRG) (Mazzone 2004). The nodose and jugular ganglia have different embryonic origins; the nodose is epibranchial placode-derived and the jugular neural crest-derived (Nasra and Belvisi 2009), and as a result, they express different growth peptides and neurotrophins (Ernfors et al. 1992; Begbie et al. 2002) and house different populations of nerve fibres. There are several known sensory nerve subtypes present in the lung, which can be identified based on adaptation indices, physiochemical sensitivity, neurochemistry, origin, myelination conduction velocities and sites of termination (Canning 2006a; Adcock et al. 2003). Of these sensory nerve subtypes, three are more mechanically sensitive; the rapidly adapting receptors (RARs), slowly adapting receptors (SARs) and the subtype known as the ‘cough’ receptor. There are also two fibre types which are more chemosensitive: C fibres and Aδ nociceptors.
Rapidly adapting receptors
RARs, or irritant receptors, are myelinated, conducting action potentials in the range of 14–23 m/s (Fox et al. 1993; Riccio et al. 1996). Although distributed throughout the airways, they are mostly found in the larger airways where they respond rapidly to mechanical stimuli such as light touch and lung hyperinflation/deflation (Mortola, Sant’Ambrogio, and Clement 1975; Sant’Ambrogio et al. 1978; Canning 2004; Nasra and Belvisi 2009). RARs are thought to be mostly nodose-derived (Riccio et al. 1996; Canning 2006) and were originally thought to be directly activated by a chemical stimulus such as capsaicin and bradykinin, although it is thought that these stimuli can indirectly activate these fibres following bronchoconstriction and mucus production (Widdicombe 2003). However, emerging evidence has suggested that a subset of RARs is responsive to capsaicin regardless of conduction velocity in naive guinea pigs (Adcock et al. 2014). Due to their mechanical sensitivity and their ability to cause cough even under anaesthesia, RARs are thought to provide the defensive cough reflex that clears the lungs from harmful particles (Nasra and Belvisi 2009; Dicpinigaitis et al. 2014).
Slowly adapting receptors
SARs are also fast-conducting, nodose-derived fibres that are sensitive to mechanical stimulation (Nasra and Belvisi 2009). However, they are less sensitive than RARs to sustained lung inflation (Schelegle and Green 2001) and are involved in tidal breathing and are important in the Hering-Breur reflex and sense when the lungs are sufficiently inflated during inspiration and initiate expiration (Schelegle 2003). SARs are not thought to play a direct role in the cough response.
The cough receptor
The more recently described cough receptors are found in the larynx, trachea and bronchi and, similarly to RARs, are nodose-derived, activated by citric acid and changes in pH, and are extremely sensitive to light punctate touch, with a low threshold for activation (Canning 2004; Nasra and Belvisi 2009). However, they differ from RARs as they have slower conduction velocities (5 m/s) and are insensitive to stretch, increased pressure, and bronchoconstriction which all activate RARs (Canning 2004; Mazzone 2004). It should be noted that the relevance of the cough receptor remains unclear in man, as the majority of work has been carried out in the anaesthetized guinea pig (Nasra and Belvisi 2009; Canning 2004; Mazzone 2004).
Aδ nociceptors
Aδ nociceptors are chemosensitive, fast-conducting myelinated fibres that differ from RARs, as they originate in the jugular ganglia and are activated by transient receptor potential vanilloid (TRPV) 1 agonists such as capsaicin; however, they do remain relatively insensitive to mechanical stimuli (Riccio et al. 1996; Kajekar et al. 1999; Yu 2005). Although they show similar effects pharmacologically to C fibres, their contribution to the cough reflex is not clear, as they are yet to be fully investigated.
C fibres
C fibres make up the majority of nerve fibres that innervate the airways (Takemura et al. 2008), are found predominantly in the airway epithelium and can originate in both the nodose and jugular ganglia. They are unmyelinated, slower conducting fibres which have a conduction velocity of ~1 m/s (Fox et al. 1993; Riccio et al. 1996; Mazzone 2004; Canning 2006). C fibres are blocked by anaesthesia and are therefore responsible for the conscious perception of airway irritants (Mazzone 2004).
C fibres are relatively less sensitive to mechanical stimuli but are activated by agonists of the TRPV1 (capsaicin, resiniferatoxin (RTX)) and transient receptor potential ankyrin (TRPA) 1 (allyl isothiocyanate (AITC), acrolein, cinnamaldehyde) ion channels, which have also been shown to cause cough in animals and in man (Coleridge and Coleridge 1984; Canning 2004; Dicpinigaitis and Alva 2005; Birrell et al. 2009; Grace et al. 2012; Dicpinigaitis et al. 2014; Adcock et al. 2014; Lalloo et al. 1995). Single-cell PCR has demonstrated the expression of TRPA1 and TRPV1 in C fibres in animals (Nassenstein et al. 2008; Brozmanova et al. 2012), and TRPV1 expression has been shown on nerves in man (Groneberg 2004). As the fibres are relatively unresponsive to mechanical stimuli, the chemical stimuli activate the nerves directly (Undem 2004; Chuaychoo et al. 2005).
C fibres are further differentiated from RARs and Aδ nociceptors by their ability to participate in local axon reflex events leading to the release of neuropeptides such as CGRP, substance P and neurokinin A (Lundberg et al. 1985; Hunter and Undem 1999; Myers, Kajekar, and Undem 2002; Baluk et al. 1992) which can evoke responses such as bronchoconstriction and mucus production (Nasra and Belvisi 2009). However, although local axon reflex events, characterized by the release of neuropeptides, have been demonstrated in rodent and guinea pig airways, it is still not clear if this happens in human airways.
There are two distinct populations of C fibres, bronchial and pulmonary C fibres, which are distinguished according to their site of termination, responsiveness to different stimuli and ganglionic origin. Bronchial C fibres are thought to innervate the extrapulmonary airways and are derived mostly from the jugular ganglia and release neuropeptides and pulmonary C fibres which innervate the lower airways (Coleridge and Coleridge 1984; Carr and Undem 2003; Undem 2004). In some cases, pulmonary C fibres have been found to be inhibitory against cough in animal models (Widdicombe and Undem 2002; Tatar et al. 1994; Chou et al. 2008), so it is thought that it is the bronchial C fibres that mediate the cough response to chemicals such as capsaicin.
Preclinical systems for assessing airway sensory nerves
Guinea pigs, dogs, cats, rabbits and rodents have all been used to investigate the neural pathways, physiology and pharmacological intervention of the cough (Hanácek et al. 1984; Gardiner and Browne 1984; Kamei et al. 1993; Tatar et al. 1994; Patel et al. 2003). However, it has been widely agreed that the guinea pig provides the most suitable animal model (Belvisi and Hele 2003), as there are a great number of similarities in the cough response between the guinea pig and man, including their sensitivity to tussive stimuli such as capsaicin and citric acid (Karlsson and Fuller 1999), they have similar lung anatomy and physiology to humans, and the lungs are also innervated similarly, unlike the mouse (Canning 2006). There are a number of experimental systems that can investigate the cough reflex both in vitro and in vivo in the guinea pig, some of which are outlined below.
Isolation of vagal ganglia
The cell bodies from airway-specific neurons in nodose and jugular ganglia can be identified and isolated for assessment. One technique involves using the retrograde tracer dye DiIC18(3),1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI). The dye is given into the airways at least 12–14 days prior to harvesting the ganglia. The tracer travels back up the neuron by retrograde transport into the cell body (Undem et al. 2002; Lieu et al. 2012; Kwong and Lee 2005). Once isolated, the airway-specific neurons can be identified under a fluorescent microscope and used in a number of ways including the following:
-
Assessment of target expression at the messenger RNA (mRNA) level (Nassenstein et al. 2008).
-
Measurement and location of the target at the protein level (Kwon et al. 2014).
-
Intracellular Ca2+ movement (i.e. using fluorescent tags (Grace et al. 2012; Dubuis et al. 2013).
-
Biochemical study (i.e. understanding signalling transduction (Hadley et al. 2014)).
-
Electrophysiological assessment (i.e. patch clamp systems (Dubuis et al. 2014))
The key advantage of this technique is that it allows both functional phenotyping and target expression in primary airway nodose and jugular ganglia (a schematic is shown in Fig. 2a). Disadvantages are that studies are performed with cell bodies rather than the nerve terminal, and as yet, it is not possible to access human primary ganglia cells for translational conformation studies.
Preclinical models of cough. a Isolation of airway-specific ganglia. Guinea pigs are intranasally dosed with the retrograde tracer dye DiI 12–14 days prior to dissection and enzymatic disassociation of the jugular and nodose ganglia. Airway-specific cells can then be identified using fluorescence imaging indicated by the overlay image, and then, cells can be used for calcium imaging or single cells taken and used for RT-PCR. b In vitro-isolated vagal nerve preparation. The vagus nerve trunk is isolated and dissected and placed into a grease gap recording chamber, where each end of the nerve is electrically and chemically isolated using Vaseline™. One end of the nerve is then attached to a recording electrode and perfused with either Krebs-Hensleitt (KH) solution or drug, and the other end of the nerve is attached to a reference electrode but bathed in KH for the duration of the experiment. When drugs are perfused, the difference between the two electrodes can be amplified and recorded as compound depolarization. The bottom panel indicates an example trace of a classical agonist/antagonist experiment; the nerve is exposed to two reproducible responses to agonist, following which the nerve is exposed to an antagonist for 10 min. The nerve is then restimulated with the agonist in the presence of the antagonist, and % inhibition of the original response can be assessed. After a brief washout period, the nerve is restimulated with agonist to ensure nerve viability. c In vivo single fibre. Guinea pigs are anaesthetized and ventilated and both cervical vagal nerves cut at the central end. The left vagus nerve is then dissected down to a single fibre and attached to a platinum recording electrode. Compounds are then aerosolized into the lungs, and firing from the single fibre is recorded. The bottom panel is an example trace of firing from a C fibre to capsaicin. d In vivo cough. Guinea pigs are placed into the two plethysmography chambers and compounds aerosolized in via a central nebulizer. The number of coughs can then be counted by a trained observer and also monitored by the Buxco Cough Analyzer software, which would show a characteristic spike (right) if a cough occurred
In vitro-isolated vagus nerve recordings
The use of the vagal trunk to assess sensory nerve activity has also been used effectively. One system involves isolation of the two ends of the vagus trunk in a grease-gap recording chamber. One end can then be challenged with stimuli/test solutions, and membrane depolarization recorded. An example of the system and traces generated is as shown in Fig. 2b and reported in various publications including (Grace and Belvisi 2011; Fox et al. 1995; Birrell et al. 2009).
This is a relatively high throughput, pharmacologically amenable method where many stimuli that activate the vagus nerve have been shown to cause cough in both animals and man (Maher et al. 2009; Patel et al. 2003). It also holds the key advantage of being able to record from human vagal tissue to provide translational data (Usmani et al. 2005; Belvisi 2008). There are, however, several limitations to consider, including the use of nerve trunk rather than the nerve terminal; the inability to specifically record from airway nerves and the recordings taken are a summation of the activity from the whole trunk, and action potential generation is not measured. Nonetheless, the recordings are robust and reproducible and have allowed the investigation of a number of tussive and antitussive compounds.
In vivo single fibre
The in vivo single-fibre recording technique allows the investigation of specific tussive or antitussive stimuli on action potential firing of single afferent airway terminating fibres (Adcock et al. 2003; Canning 2004). This technique measures the effects of compounds on the nerve termini in the whole animal and is therefore more physiologically relevant than the isolated vagus or ganglia. Figure 2c shows a representative scheme and trace from one model system. Fibre type can be identified using a range of criteria (Adcock et al. 2003). However, the disadvantage of this technique is that it is relatively low throughput, requires relatively large amounts pharmacological/biological tools with appropriate characteristics for in vivo work and is extremely technically demanding.
In vivo cough recordings
The most common model of cough is the conscious guinea pig cough model. This often involves placing conscious animals into individual Perspex chambers where they can be exposed to tussive agents (Fig. 2d). Coughs can be counted by both automated cough counting software which uses an custom-designed algorithm to recognize cough (Lewis et al. 2007), measurement of airflow and assessment by a trained observer who will recognize both the sound and characteristic change of posture adopted by the animal (Nasra and Belvisi 2009; Andrè et al. 2009; Birrell et al. 2009; Lee et al. 2006; Grace et al. 2012; Maher et al. 2009; Trevisani et al. 2004). The key advantage of this technique is that studies have shown that tussive responses induced in guinea pigs closely resemble that seen in man (Laude, Higgins, and Morice 1993; Birrell et al. 2009; Lee et al. 2006); however, this model does not allow the identification of the fibre type responsible and requires large amounts of test compound.
Disease models
Much of the research into the cough response has been carried out in naive animals and healthy cells/tissue, but it is known that the cough reflex and presumably sensory nerve activity are altered in disease. Therefore, investigating sensory nerves under disease conditions is vital. Generally, the approach used is to model the disease in the animal and study how the sensory nerve phenotype has been altered. One example is the modelling of COPD, a disease where the patients are known to cough more and be more sensitive to inhaled tussive agents including the TRPV1 agonist capsaicin (Sumner et al. 2013). To model COPD, researchers expose the guinea pigs to the main causative agent linked to the development of COPD, cigarette smoke (CS). The exposed guinea pigs are then shown to be more sensitive to tussive stimuli (Karlsson et al. 1991b; Lewis et al. 2007; Wortley et al. 2011; Bergren 2001; Karlsson et al. 1991a). Furthermore, tissue and ganglia harvested from the CS-exposed guinea pigs have an altered phenotype. These data will be discussed in greater detail later.
Measuring cough in the clinic
Although guinea pigs have similar pharmacology and physiology to the human airways, there are several caveats to using animal models (Morice et al. 2007a, b), and therefore, cough models may not always translate to the clinical situation. Alongside animal models, cough can also be measured in the clinic in both healthy and diseased patients. There are three main techniques used in the clinic to monitor cough and to assess the validity of specific antitussive therapies. These are subjective quality of life questionnaires, ambulatory cough counting and cough reflex sensitivity testing.
Cough questionnaires
Specialist qualitative cough questionnaires have been established that measure the impact of chronic cough on daily life. They provide a subjective retrospective analysis of cough severity (Nasra and Belvisi 2009). There are currently three questionnaires in common use that look at cough as a primary end point and are used it to assess the impact of cough on quality of life. These are the Leicester Cough Questionnaire (LCQ) (Birring et al. 2003), where a lower score indicates a poorer quality of life, and the Cough Quality of Life Questionnaire (CQLQ) (French et al. 2002) and Chronic Cough Impact Questionnaire (CCIQ) (Baiardini et al. 2005), where a higher score indicates a lower quality of life. All questionnaires measure the physical, psychological and social impact of cough using a specialized scoring system and are both validated and comparable in establishing the severity of cough.
Ambulatory cough counting
Cough monitors provide a non-invasive method of measuring cough over a 24-h time period (Kelsall et al. 2011). This is a more representative measure of the symptom of cough, as it does not rely upon patient recall and is carried out in the patient’s natural environment, rather than under laboratory conditions (Smith 2010). Patients wear a discrete recording device equipped with a microphone which records cough sounds over a set time period (Smith and Woodcock 2008; Smith 2010).
There are currently several systems in use, but none are commercially available at present; the LifeShirt (VivoMetrics Incorporate, Ventura, CA) (Coyle et al. 2005; Smith 2010) and the CoughCOUNT (KarmelSonix, Haifa, Israel) (Vizel et al. 2010) are no longer in production. The other systems in use include the Leicester Cough Monitor, which, although widely published, is not yet fully automated (Birring et al. 2008) (McGuinness et al. 2008). The sensitivity of the system is most recently reported as 83.8 % in patients (n = 12) and 82.3 % in healthy controls (n = 8) (Yousaf et al. 2013) on pooled data; how the sensitivity varies between individual subjects is not reported. The Hull Automated Cough Counter which uses neural network-based technology to recognize sounds is still in development (Barry et al. 2006). The final cough counting system is the VitaloJAK (Vitalograph Limited, Buckinghamshire, UK), which uses a custom-made digital recording device to record cough through sounds detected by microphones on the chest wall and lapel (Smith and Woodcock 2008). This monitoring system no longer requires calibration (Smith 2010) and consists of a compression algorithm that removes silence and the bulk of non-cough sounds from 24-h acoustic recordings. This provides a sound file an average of 85 min in length containing median 100.0 % (range 97.3–100 %) of coughs in the original 24-h recording when tested on a group of 47 subjects, including chronic cough, asthma, COPD, lung cancer and healthy controls (McGuinness et al. 2012 and unpublished data).
Cough reflex sensitivity
Capsaicin and citric acid are the most common agonists used to induce cough in the clinic, and in the case of capsaicin, this is because it has been shown to have no adverse effects (Dicpinigaitis and Alva 2005) and induces cough in a dose-dependent, reproducible manner (Dicpinigaitis 2007). Other tussive agents include bradykinin, prostaglandins, fog and hypertonic saline (Nasra and Belvisi 2009). This technique involves the inhalation of irritants and counting the number of coughs over a set time period, which allows a comparison between healthy and diseased individuals (Nasra and Belvisi 2009).
The most common method of measuring cough reflex sensitivity is using the C2 and C5 parameters, which is the concentration of tussive stimulus (commonly capsaicin) required to reach two or five coughs (Doherty et al. 2000; Dicpinigaitis 2006; Morice et al. 2007a, b), reached by inhaling single doubling doses of stimulus (Hilton et al. 2013). This technique has been found to be highly reproducible (Dicpinigaitis 2003) and easy to perform (Morice et al. 2007a, b), and clinicians can discern differences between disease groups.
Recently, however, the use of C2 and C5 parameters has come into question, as they have been shown to only weakly correlate with the spontaneous cough frequency measured using ambulatory 24-h cough counts (Hilton et al. 2013; Decalmer et al. 2007; Birring et al. 2006). Instead, a new capsaicin challenge has been put forward using non-linear mixed effects dose-response modelling, which uses EMax or ED50 rather than C2 and C5 to distinguish between health and disease, which correlates well with cough frequency (Hilton et al. 2013). This has also been shown to be more similar to conscious cough in animals (Smith et al. 2012).
TRP channels in cough
Airway sensory nerves are known to express a variety of receptors and ion channels that are activated by a variety of exogenous and endogenous mediators to cause cough. A greater understanding of the mechanisms of how these receptors and ion channels are involved in cough may lead to the development of new and effective therapies. In this part of the review, the role of several members of the TRP family of ion channels, TRPV1, TRPA1, TRPV4 and transient receptor potential melastatin (TRPM) 8, in the activation of airway sensory nerves and cough is outlined.
The TRP superfamily of ion channels is a set of cation-dependent transmembrane proteins which show a preference for Ca2+, initially discovered in the Drosophila fly and so named because of their transient response to bright light (Montell and Rubin 1989; Ramsey et al. 2006; Caterina et al. 1997). TRP channels are known as cellular sensors (Clapham 2003), as they respond to changes including temperature, stretch, chemicals, oxidation, osmolarity and pH (Picazo-Juárez et al. 2011; Moran et al. 2011) in the cellular environment. They are also sensors for certain herbs, spices, venoms and toxins (Vriens et al. 2008). This family of ion channels has 28 members in six subfamilies (TRPC, TRPV, TRPM, TRPA, TRPP, TRPML) based on sequence homology and amino acid sequence (Clapham 2003), and all have six transmembrane domains with the pore situated between domains 5 and 6, variable degrees of ankyrin repeats and an intracellular N and C terminus (Caterina et al. 1997; Ramsey et al. 2006). Functional channels are mostly formed as homotetramers, but heteroteteramization can also occur (Latorre et al. 2009; Cheng et al. 2010). Activation of TRP channels allows cations to pass through the channel on the membrane, causing depolarization of the cells and causing a wide range of cellular responses (Kaneko and Szallasi 2014).
As cellular sensors, TRP channels are therefore linked to sensory perception, and many are associated with the pathogenesis of several respiratory diseases including COPD, asthma, cancer and cystic fibrosis (Caterina et al. 1997; Arniges et al. 2004; Nilius 2007; Zhu et al. 2009). Members of the TRP family of ion channels have been outlined as strong candidates for sensory receptors that modulate cough in humans (reviewed in Grace et al. 2014b; Grace et al. 2013; Materazzi et al. 2009), and there is a large body of evidence outlining the role of TRPV1 and TRPA1 in cough and evidence indicating that both TRPV4 and TRPM8 could also play a role.
TRPV1
The receptor for the tussive stimulus capsaicin was identified to be the vanilloid receptor TRPV1 (Szallasi and Blumberg 1990; Caterina et al. 1997), and this was the first TRP channel to be identified as a key regulator of tussive reflexes (Lalloo et al. 1995). TRPV1 is a polymodal ion channel which is activated by a diverse range of stimuli including irritant chemicals (i.e. capsaicin), low pH, increases in temperature (around 43 °C) and a number of endogenous mediators (Jordt et al. 2000; Moriyama et al. 2005; Zhou et al. 2011; Jia et al. 2002; Kagaya et al. 2002; Zygmunt et al. 1999; Caterina et al. 1997; Carr and Undem 2003; Kollarik and Undem 2004; Hwang et al. 2000). In addition, activation of certain G protein-coupled receptors (GPCRs) by inflammatory mediators has been shown to result in the opening of TRPV1 ion channels. These include bradykinin (Grace et al. 2012), PGE2 (Grace et al. 2012; Moriyama et al. 2005) and peptides which activate protease-activated receptors (PARs) (Amadesi et al. 2004; Gatti et al. 2006). Table 1 contains a summary of some of the reported TRPV1 agonists (direct and indirect activators) and antagonists.
TRPV1 expression
TRPV1 mRNA has been shown to be expressed in vagal ganglia (Caterina et al. 1997; Ahluwalia et al. 2000; Ichikawa and Sugimoto 2004), with single-cell reverse transcription (RT)-PCR showing TRPV1 in airway neurons (Nassenstein et al. 2008; Lieu et al. 2012). As with all the TRP receptors, it is currently difficult to determine expression of TRPV1 at the protein level because of the lack of selective antibodies for accurate staining although papers describing protein expression have been published. Instead, specific ligands can be used to trigger a functional response (i.e. Ca2+ flux, nerve depolarization and firing) to demonstrate the presence of the ion channels. Using this approach, it has been shown that TRPV1 is functionally expressed on naive guinea pig airway jugular neurons with less activation by TRPV1 ligands on the nodose neurons (Grace et al. 2012; Hu et al. 2014). Interestingly, in a cigarette smoke (CS)-driven model system and also following allergen exposure, it has been shown that the nodose ganglia cells become much more responsive to capsaicin, suggesting a phenotype change in disease (Wortley et al. 2011; Lieu et al. 2012). Indeed, others have reported that the expression of TRPV1 ion channels on sensory nerves is increased in patients with idiopathic cough (Groneberg 2004). Furthermore, two independent studies in eight European countries have identified six TRPV1 single-nucleotide polymorphisms (SNPs) associated with a higher risk for chronic cough (Smit et al. 2012).
TRPV1 activation
A number of TRPV1 ligands including capsaicin and RTX have been shown to activate isolated animal vagal tissues (Maher et al. 2009; Grace et al. 2012; Birrell et al. 2014; Fox et al. 1995). As discussed above, parallel data is reported in vagal tissue harvested from human lungs, thus providing translational evidence (Birrell et al. 2009; Birrell et al. 2014; Usmani et al. 2005). Furthermore, using this technique, it has been shown that under disease conditions, vagal tissue exhibits enhanced responses to TRPV1 agonists following CS exposure (Wortley et al. 2011).
Initially, it was thought that only C fibres expressed functional TRPV1 ion channels (Geppetti et al. 2006); however, using techniques like in vivo single airway fibre recordings, it is becoming clear that capsaicin can cause action potentials in a subset of RAR Aδ fibres regardless of conduction velocity in naive animals (Adcock et al. 2014). We wait to see if this profile of TRPV1 responses is altered under disease conditions.
It is established that the numbers of coughs to inhaled capsaicin are increased in disease models (Lewis et al. 2007; Maher and Belvisi 2010; Carr et al. 2002; Myers, Kajekar, and Undem 2002; Karlsson et al. 1991a; Wortley et al. 2011). It is well known that capsaicin causes coughing in guinea pigs and man (Lalloo et al. 1995; Fox et al. 1993; Caterina et al. 1997; Nasra and Belvisi 2009; Riccio et al. 1996); indeed, it is often the tussive agent of choice when performing clinical trials/cough sensitivity assessment (discussed in greater detail previously). Other agents that trigger cough such as citric acid/low pH, PGE2 and bradykinin do so at least partially through via the TRPV1 ion channel (Fuller and Choudry 1987; Morice et al. 2007a, b; Karlsson and Fuller 1999; Doherty et al. 2000; Laude, Higgins, and Morice 1993; Lalloo et al. 1995; Morice, Kastelik, and Thompson 2001; Kollarik and Undem 2002; Maher et al. 2009; Grace et al. 2012). This data indicates that TRPV1 is central to cough responses in guinea pigs and man. Furthermore, as a number of clinical studies have shown a heightened cough response to inhaled capsaicin in patients with disease such as COPD and idiopathic cough (Choudry and Fuller 1992; Groneberg 2004; Nieto et al. 2003; Prudon et al. 2005; Ternesten-Hasséus et al. 2006; Sumner et al. 2013), the fact that many of the TRPV1 ligands are altered in a diseased airway (i.e. pH) implicates TRPV1 in enhanced troublesome cough.
TRPV1 antagonists in the clinic
Several TRPV1 antagonists have been developed and profiled in clinical trials; however, most of those that have entered clinical trials cause hyperthermia as a side effect. In both animal and human studies, the use of TRPV1 antagonists is known to cause hyperthermia and impaired perception of noxious heat which is clearly an issue for potential development compounds (Gavva et al. 2007; Gavva et al. 2008; Eid 2011; Krarup et al. 2011). Antagonizing TRPV1 with certain compounds has been shown to raise the core body temperature of the patient to an unacceptable level and also impairs noxious heat sensation raising the probability of scalding injuries (Preti et al. 2012). Nevertheless, several small-molecule TRPV1 antagonists have been developed that have overcome these problems which are outlined below.
In 2005, Janssen released a novel TRPV1 antagonist JNJ17203212 which was shown to be highly selective and well tolerated and also significantly inhibited capsaicin-induced cough to the same level as codeine (Bhattacharya et al. 2007). However, development into the clinic was inhibited because of hyperthermia (Swanson et al. 2005). More recently, JNJ39729209 has been developed which also inhibited capsaicin-induced cough in guinea pigs and had a low clearance rate and good orally bioavailability (Maher et al. 2011). In addition, this compound seems to have circumvented the problem of hyperthermia, as it only showed a small increase in core temperature (1 °C) at a fully efficacious dose (Maher et al. 2011). This is yet to be taken into the clinic, but following the promising preclinical data, it would be interesting to see how it would fare at inhibiting capsaicin-induced cough reflex sensitivity in proof of mechanism studies and spontaneous coughing in patients with chronic cough.
A study was recently published profiling the impact of a TRPV1 inhibitor, SB 705498 (Gunthorpe and Chizh 2009), on both capsaicin cough sensitivity and spontaneous cough responses in patients with refractory chronic cough (Khalid et al. 2014). SB 705498 also appears to be devoid of hyperthermia issues and is rapidly absorbed with a long elimination half-life of 50–60 h (Chizh and Sang 2009). The study used both capsaicin cough challenge and 24-h ambulatory cough monitoring. However, SB 705498 caused a very small shift in the capsaicin-induced cough response curve and had no effect on spontaneous cough (Khalid et al. 2014). This could suggest that TRPV1 is not involved in idiopathic cough or more likely that this compound did not possess the optimum efficacy profile or pharmacokinetic characteristics to fully engage the target. Whatever the case, this data does not rule out a role for TRPV1 in coughing in other conditions such as increased cough in basal COPD and during exacerbations. Indeed a phase II clinical trial (double-blind, randomized, placebo-controlled) is ongoing in COPD patients with chronic cough with a highly potent TRPV1 inhibitor, XEN-DO501. The primary endpoint is objective cough monitoring in chronic cough associated with COPD together with a proof of target engagement by including a capsaicin challenge in the protocol. This compound has undergone phase I clinical studies where it has been shown to be safe and well tolerated and has no significant hyperthermia issues (Round et al. 2011). Preclinical studies have demonstrated that XEN -DO501 inhibited capsaicin-induced cough in naive animals and also inhibited a heightened capsaicin cough response in guinea pigs exposed to CS (Wortley et al. 2014).
TRPA1
TRPA1 is a voltage-dependent Ca2+ permeable cation channel, where the major mode of activation is through covalent modification of N-terminal cysteines or lysines by electrophilic compounds (Bandell et al. 2004; Banner et al. 2011; Hinman et al. 2006). TRPA1 was initially identified as a noxious cold sensor and a mechanosensor (Story et al. 2003), but it has since been shown to be activated by a wide range of pungent natural and environmental irritants which cause pain and inflammation (Bandell et al. 2004; Dicpinigaitis et al. 2014; Macpherson et al. 2005; Bautista, Pellegrino, and Tsunozaki 2013). The number of activators of TRPA1 far exceeds those for any other TRP channels. Similarly to TRPV1, it can also be activated by the indirect endogenous mediators PGE2 and bradykinin (Grace et al. 2012; Bautista et al. 2006), and activation of PAR2 also leads to activation of TRPA1 (Dai et al. 2007; Terada et al. 2013). A number of agonists and antagonists for the TRPA1 channel are summarized in Table 1.
TRPA1 expression
TRPA1 is expressed in a subset of TRPV1 expressing small-diameter nociceptive neurons (Story et al. 2003; Bautista 2005), and single-cell PCR has indicated that TRPA1 mRNA levels are found in airway vagal neurons (Nassenstein et al. 2008; Jang et al. 2012). TRPA1 has also been reported to be expressed in a number of non-neuronal cell types including epithelial cells, airway smooth muscle cells and fibroblasts (Jaquemar et al. 1999; Kunert-Keil et al. 2006; Stokes et al. 2006; Mukhopadhyay et al. 2011). A functional response in neurons has also been demonstrated as TRPA1 ligands induce calcium release in airway-specific jugular ganglia cells (Grace et al. 2012). It is not yet known if expression levels of TRPA1 are altered in disease conditions, despite the fact that many CS components including acrolein and crotonaldehyde activate TRPA1 (Bautista et al. 2006; Andrè et al. 2008; Andrè et al. 2009; Lin et al. 2010; Volpi et al. 2011). However, Smit et al. have reported that there were no TRPA1 SNPs which associate smoking or occupational exposure with cough although this does not rule out changes in protein expression or the activation status of the channel (Smit et al. 2012).
TRPA1 activation
TRPA1 is the molecular target for by-products of oxidative stress including reactive oxygen species (ROS) and other electrophilic compounds, including hypochlorite, hydrogen peroxide and nitric oxide (Bautista 2005; Bessac et al. 2008; Takahashi et al. 2011; Takahashi et al. 2008; Sawada et al. 2008; Andersson et al. 2008). TRPA1 activation leads to activation of vagal bronchopulmonary C fibres in rodent lungs (Bessac et al. 2008; Taylor-Clark et al. 2008; Nassenstein et al. 2008; Birrell et al. 2009; Taylor-Clark et al. 2009; Andrè et al. 2009; Grace et al. 2012) and cough in both animals and human volunteers (Birrell et al. 2009; Andrè et al. 2009)
A number of TRPA1 ligands including acrolein, cinnamaldehyde and AITC have been shown to cause depolarization in an in vitro model of sensory nerve activation (Birrell et al. 2009; Grace et al. 2012), with translational data in human tissue (Birrell et al. 2009; Grace et al. 2012). Unlike the TRPV1 agonist capsaicin, acrolein appears to only activate C fibres and had no effect on Aδ fibres in a guinea pig model of airway single-fibre activation (Adcock et al. 2014).
Acrolein, cinnamaldehyde, crotonaldehyde and AITC have been shown to cause cough in conscious naive guinea pigs (Birrell et al. 2009; Caceres et al. 2009; Andrè et al. 2009; Brozmanova et al. 2012), and in addition, CS itself is known to cause cough through activation of TRPA1 (Andrè et al. 2009). Furthermore, cinnamaldehyde has been shown to induce a concentration-dependent cough response in human volunteers (Birrell et al. 2009). Unlike TRPV1 agonists, responses to TRPA1 agonists have not yet been shown to be enhanced in disease states.
TRPA1 antagonists in the clinic
Selective TRPA1 inhibitors are also potential therapeutic agents for chronic cough, as TRPA1 plays an important role in respiratory symptoms induced by endogenous and exogenous irritants and is also a sensor of oxidative stress. In addition, TRPA1 antagonists have not yet been shown to have the same temperature regulation safety concerns as TRPV1, which would suggest that, perhaps, this channel is a more suitable target. However, despite this, currently, there is only one TRPA1 channel antagonist in the clinic to combat chronic cough, although several are in preclinical development outlined below for potential use in cough and also asthma.
Cubist pharmaceuticals have developed a novel small-molecule TRPA1 antagonist CB-189625, which is currently in phase I clinical trials in the Netherlands used to target acute pain and inflammatory conditions (Preti et al. 2012). Janssen also has a compound in the preclinical stage of testing, and this has been suggested to have antitussive activity (Preti et al. 2012). However, the only antagonist currently in clinical trials for the treatment for respiratory disorders is GRC17356 by Glenmark pharmaceuticals. Inhalation studies are planned to be initiated in both healthy and asthmatic volunteers in an allergen challenge model (Preti et al. 2012) and have recently been entered into a 4-week phase II study to evaluate efficacy, safety and tolerability of inhaled GRC17356 in patients with refractory chronic cough. It has already been shown to inhibit citric acid-induced cough in guinea pigs (Mukhopadhyay et al. 2014) and has also shown positive data in a phase 2a proof of concept study in patients with painful diabetic neuropathy.
TRPA1 also activates non-neuronal cells including fibroblast epithelial cells and airway smooth muscle, which can also add to the cough response. Activation of TRPA1 on these cells has been shown to trigger the release of inflammatory mediators (Keatings et al. 1996; Crooks et al. 2000; Gompertz et al. 2001; Mukhopadhyay et al. 2011). Many inflammatory mediators are endogenous TRPA1 agonists, including ROS, prostaglandins and bradykinin (Grace and Belvisi 2011). Therefore, blocking TRPA1 would not only have an effect on nerves, but also aid to block inflammation caused by activating TRPA1 on non-neuronal cells which can also lead to a reduction in cough response.
TRPV4
TRPV4 is a Ca2+ permeable polymodal receptor with a proline-rich region and six ankyrin repeats within its cytosolic N terminus (Nilius and Szallasi 2014). TRPV4 is activated by moderate temperatures (>24 °C) and was originally characterized as a sensor of osmotic stress (Strotmann et al. 2000; Suzuki et al. 2003; Liedtke and Friedman 2003), as TRPV4 knockout mice show abnormalities with systemic osmotic and external somatosensory stimuli (Liedtke et al. 2000). Similarly to TRPA1, TRPV4 is also linked to the GPCR PAR2, which has been shown to sensitize TRPV4 in DRG neurons and amplify neurogenic inflammation and responses to painful stimuli (Alessandri-Haber et al. 2006; Grant et al. 2007). In addition, PAR2 stimulation has been shown to activate TRPV4 channels (Poole et al. 2013), where PAR2 activation leads to receptor-operated gating of TRPV4 (Poole et al. 2013; Grace et al. 2014a). A number of selective ligands for the TRPV4 channel are shown in Table 1.
Tissue expression
TRPV4 is expressed in neurons of both the central and peripheral nervous systems including expression in DRG neurons (Grant et al. 2007) and at the mRNA level in pulmonary sensory neurons (Ni et al. 2006). TRPV4 has been shown to be functionally present on airway ganglia, and contrary to both TRPV1 and TRPA1 ligands, TRPV4 ligands were shown to induce calcium signal in the nodose ganglia but had no effect in the jugular ganglia (Belvisi et al. 2013).
Activation of TRPV4
The small-molecule TRPV4 activator GSK1016790a has been shown to induce depolarization of the isolated vagus nerve in both guinea pig and human tissue and also caused cough which was blocked by the selective antagonist HC067047 in conscious naive guinea pigs (Belvisi et al. 2013). This was suggested to be through activation of mechanosensitive Aδ fibres (Belvisi et al. 2013; Adcock et al. 2014). There are currently no studies in man with TRPV4 ligands; however, hypotonic solution, which is known to activate TRPV4 (Strotmann et al. 2000; Liedtke et al. 2000; Jia et al. 2004), has been reported to case cough and bronchoconstriction in asthmatic subjects (Schoeffel et al. 1981; Fuller and Collier 1984). Furthermore, distilled water, or fog, another hypotonic solution, is also used as a tussive agent in the clinic (Morice et al. 2007a). The role of TRPV4 in airway afferents requires further investigation; however, this could provide a novel mechanism for activation of sensory nerves and cough.
TRPV4 antagonists in the clinic
As not much is known about the role of TRPV4 in initiating cough, there are currently no antagonists in the clinic for the treatment of cough or respiratory disorders. However, it has been suggested that TRPV4 does cause cough via a different mechanism to that seen with TRPA1 and TRPV1 (Belvisi et al. 2013). In addition, activation of TRPV4 has been shown to cause contraction of both human and animal airway smooth muscle (Jia et al. 2004; McAlexander et al. 2014). Therefore, a TRPV4 antagonist could prove to be an attractive target for lung disease to combat both bronchoconstriction and cough and could be used alongside a TRPA1 or TRPV1 antagonist to target a different population of airway afferent fibres.
TRPM8
The TRPM8 channel is a thermosensor activated by physiological cool temperatures between 15 and 28 °C (McKemy et al. 2002; Peier et al. 2002). Direct activators of the channel are compounds which elicit a cooling sensation such as menthol, icilin and eucalyptol (Peier et al. 2002; Zhou et al. 2011). The first agonist to be identified was the monoterpene menthol, where the molecular mechanism of its pharmacological activity was determined to be through TRPM8 (McKemy et al. 2002). However, menthol has also been shown to show some biological activity at the TRPA1 channel, where low concentrations of menthol were shown to activate the channel and high concentrations were able to block TRPA1 (Karashima et al. 2007). Table 1 contains a number of agonists and antagonists for TRPM8. The channel functions as a homotetramer (Nilius and Szallasi 2014) and is composed of four identical subunits with six transmembrane domains (Latorre et al. 2007).
Tissue expression
The TRPM8 channel is expressed in a subpopulation of cold responsive primary afferent neurons within the dorsal root and trigeminal ganglia which are different to the neurons which express TRPV1 and TRPA1 (Clapham et al. 2001; Peier et al. 2002; Story et al. 2003). TRPM8 is expressed in cold-sensitive afferents expressed in the upper and lower airways (Abe et al. 2005; Xing et al. 2008; Zhou et al. 2011; Keh et al. 2011), including vagal sensory neurons (Xing et al. 2008; Nassenstein et al. 2008). Single-cell PCR has indicated that TRPM8 is expressed in around 60 % of nasal trigeminal neurons and is also expressed on retrogradely labelled jugular neurons (Hondoh et al. 2010; Plevkova et al. 2013).
Activation of TRPM8
Although TRPM8 has been suggested to be responsible for cough and bronchoconstriction caused by inhalation of cold air (Peier et al. 2002; Xing et al. 2008), there are a number of animal studies which indicate the antitussive properties of the TRPM8 agonist menthol. Inhalation of menthol vapour reduced citric acid-induced cough by 50 % in guinea pigs (Laude et al. 1994), and inhibition of citric acid-induced cough was also indicated in both conscious and anaesthetized guinea pigs (Plevkova et al. 2013). Menthol was shown to inhibit CS-induced airway irritancy in mice, and this inhibition was reversed by the selective TRPM8 antagonist AMTB (Willis et al. 2011). Menthol’s antitussive activity was suggested to be through activation of TRPM8 channels present on nasal trigeminal afferent neurons, which do not express TRPA1 or TRPV1 (Plevkova et al. 2013).
In man, citric acid-induced cough was reduced in healthy volunteers after inhalation of menthol compared to air or pine oil controls (Morice et al. 1994), and menthol vapour caused a short-lasting decrease in capsaicin-induced cough in normal subjects (Wise et al. 2012). In addition, nasal application of menthol has been shown to inhibit capsaicin-induced cough in man (Buday et al. 2012).
TRPM8 antagonists in the clinic
Contrary to activation of TRPA1, TRPV1 and TRPV4, activation of TRPM8 by menthol has instead been shown to have an antitussive effect (Laude et al. 1994; Wise et al. 2012). Menthol has been used for years in a number of OTC therapies, for its antitussive properties (Al Aboud 2010). In addition, menthol has been added to cigarettes for a number of years to reduce airway irritancy (reviewed in (Nilius and Szallasi 2014). If menthol is anti-tussive via its sctivity at TRPM8 thenantagonizing TRPM8 may not be a suitable therapeutic target for the treatment of chronic cough.
Conclusions
In summary, the treatment of chronic cough is currently an urgent unmet medical need. Current therapies have proved ineffective or have side effect liabilities, but novel therapeutics are in development, some of which are in clinical studies, which target sensory afferents and the peripheral arm of the reflex, which may show greater efficacy. TRP channels, present on the vagal sensory nerves, have been shown to play a major role in the tussive response in both animals and man, and therefore, targeting these channels could provide novel antitussive medications. TRPV1 remains an attractive target for treatment, and currently, there are a number of compounds undergoing clinical trials for efficacy in patients with chronic cough. There is also emerging evidence that other members of this family could play a significant role in activation of airway sensory nerves and cough which may provide future therapeutic options.
References
Abe J, Hosokawa H, Okazawa M et al (2005) TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res Mol Brain Res 136:91–98. doi:10.1016/j.molbrainres.2005.01.013
Adcock JJ, Douglas GJ, Garabette M et al (2003) RSD931, a novel anti-tussive agent acting on airway sensory nerves. Br J Pharmacol 138:407–416. doi:10.1038/sj.bjp.0705056
Adcock JJ, Birrell MA, Maher SA et al (2014) Making sense of sensory nerves: an in vivo characterisation of Aδ- and C-fibres innervating guinea-pig airways. AJRCCM A3969
Ahluwalia J, Urban L, Capogna M et al (2000) Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience 100:685–688, PMID: 11036202
Al Aboud K (2010) The founder of Vicks: Lunsford Richardson (1854-1919). Skinmed 8:100–101, PMID: 20527143
Alessandri-Haber N, Dina OA, Joseph EK et al (2006) A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J Neurosci 26:3864–3874. doi:10.1523/JNEUROSCI. 5385-05.2006
Amadesi S, Nie J, Vergnolle N et al (2004) Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci 24:4300–4312. doi:10.1523/JNEUROSCI. 5679-03.2004
Andersson DA, Gentry C, Moss S, Bevan S (2008) Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci 28:2485–2494. doi:10.1523/JNEUROSCI. 5369-07.2008
Andrè E, Campi B, Materazzi S et al (2008) Cigarette smoke-induced neurogenic inflammation is mediated by α, β-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 118:2574–2582. doi:10.1172/JCI34886
Andrè E, Gatti R, Trevisani M et al (2009) Transient receptor potential ankyrin receptor 1 is a novel target for pro-tussive agents. Br J Pharmacol 158:1621–1628. doi:10.1111/j.1476-5381.2009.00438.x
Arniges M, Vázquez E, Fernández-Fernández JM, Valverde MA (2004) Swelling-activated Ca2+ entry via TRPV4 channel is defective in cystic fibrosis airway epithelia. J Biol Chem 279:54062–54068. doi:10.1074/jbc.M409708200
Baiardini I, Braido F, Fassio O et al (2005) A new tool to assess and monitor the burden of chronic cough on quality of life: Chronic Cough Impact Questionnaire. Allergy 60:482–488. doi:10.1111/j.1398-9995.2005.00743.x
Baluk P, Nadel JA, McDonald DM (1992) Substance P-immunoreactive sensory axons in the rat respiratory tract: a quantitative study of their distribution and role in neurogenic inflammation. J Comp Neurol 319:586–598. doi:10.1002/cne.903190408
Bandell M, Story GM, Hwang SW et al (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41:849–857. doi:10.1016/S0896-6273(04)00150-3
Banner KH, Igney F, Poll C (2011) TRP channels: emerging targets for respiratory disease. Pharmacol Ther 130:371–384. doi:10.1016/j.pharmthera.2011.03.005
Barry SJ, Dane AD, Morice AH, Walmsley AD (2006) The automatic recognition and counting of cough. Cough 2:8. doi:10.1186/1745-9974-2-8
Bautista DM (2005) Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci 102:12248–12252. doi:10.1073/pnas.0505356102
Bautista DM, Jordt S-E, Nikai T et al (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124:1269–1282. doi:10.1016/j.cell.2006.02.023
Bautista DM, Pellegrino M, Tsunozaki M (2013) TRPA1: a gatekeeper for inflammation. Annu Rev Physiol 75:181–200. doi:10.1146/annurev-physiol-030212-183811
Begbie J, Ballivet M, Graham A (2002) Early steps in the production of sensory neurons by the neurogenic placodes. Mol Cell Neurosci 21:502–511. doi:10.1006/mcne.2002.1197
Behrendt H-J, Germann T, Gillen C et al (2004) Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol 141:737–745. doi:10.1038/sj.bjp.0705652
Belvisi MG (2008) Preclinical assessment of novel therapeutics on the cough reflex: cannabinoid agonists as potential antitussives. Lung 186(Suppl):S66–S69. doi:10.1007/s00408-007-9028-8
Belvisi MG, Geppetti P (2004) Cough. 7: Current and future drugs for the treatment of chronic cough. Thorax 59:438–440. doi:10.1136/thx.2003.013490
Belvisi MG, Hele DJ (2003) Soft steroids: a new approach to the treatment of inflammatory airways diseases. Pulm Pharmacol Ther 16:321–325. doi:10.1016/S1094-5539(03)00105-6
Belvisi MG, Dubuis E, Birrell MA (2011) Transient receptor potential A1 channels: insights into cough and airway inflammatory disease. Chest 140:1040–1047. doi:10.1378/chest. 10-3327
Belvisi MG, Bonvini SJ, Grace MS et al (2013) Activation of airway sensory nerves: a key role for the TRPV4 channel. Am J Respir Crit Care Med 187:A5265
Bergren DR (2001) Chronic tobacco smoke exposure increases cough to capsaicin in awake guinea pigs. Respir Physiol 126:127–140. doi:10.1016/S0034-5687(01)00193-1
Bessac BF, Sivula M, von Hehn CA et al (2008) TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 118:1899–1910. doi:10.1172/JCI34192
Bhattacharya A, Scott BP, Nasser N et al (2007) Pharmacology and antitussive efficacy of 4-(3-trifluoromethyl-pyridin-2-yl)-piperazine-1-carboxylic acid (5-trifluoromethyl-pyridin-2-yl)-amide (JNJ17203212), a transient receptor potential vanilloid 1 antagonist in guinea pigs. J Pharmacol Exp Ther 323:665–674. doi:10.1124/jpet.107.127258
Bianco S, Vaghi A, Robuschi M, Pasargiklian M (1988) Prevention of exercise-induced bronchoconstriction by inhaled frusemide. Lancet 2:252–255. doi:10.1016/S0140-6736(88)92540-8
Birrell MA, Belvisi MG, Grace M et al (2009) TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med 180:1042–1047. doi:10.1164/rccm.200905-0665OC
Birrell MA, Bonvini SJ, Dubuis E et al (2014) Tiotropium modulates transient receptor potential V1 (TRPV1) in airway sensory nerves: a beneficial off-target effect?⋆. J Allergy Clin Immunol. doi:10.1016/j.jaci.2013.12.003
Birring SS, Prudon B, Carr AJ et al (2003) Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 58:339–343. doi:10.1136/thorax.58.4.339
Birring SS, Matos S, Patel RB et al (2006) Cough frequency, cough sensitivity and health status in patients with chronic cough. Respir Med 100:1105–1109. doi:10.1016/j.rmed.2005.09.023
Birring SS, Fleming T, Matos S et al (2008) The Leicester Cough Monitor: preliminary validation of an automated cough detection system in chronic cough. Eur Respir J 31:1013–1018. doi:10.1183/09031936.00057407
Bolser DC (2006) Cough suppressant and pharmacologic protussive therapy: ACCP evidence-based clinical practice guidelines. Chest 129:238S–249S. doi:10.1378/chest.129.1_suppl.238S
Brignall K, Jayaraman B, Birring SS (2008) Quality of life and psychosocial aspects of cough. Lung 186(Suppl):S55–S58. doi:10.1007/s00408-007-9034-x
Brozmanova M, Plevkova J, Tatar M, Kollarik M (2008) Cough reflex sensitivity is increased in the guinea pig model of allergic rhinitis. J Physiol Pharmacol 59(Suppl 6):153–161, PMID: 19218639
Brozmanova M, Mazurova L, Ru F et al (2012) Comparison of TRPA1-versus TRPV1-mediated cough in guinea pigs. Eur J Pharmacol 689:211–218. doi:10.1016/j.ejphar.2012.05.048
Buday T, Brozmanova M, Biringerova Z et al (2012) Modulation of cough response by sensory inputs from the nose - role of trigeminal TRPA1 versus TRPM8 channels. Cough 8:11. doi:10.1186/1745-9974-8-11
Caceres AI, Brackmann M, Elia MD et al (2009) A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci 106:9099–9104. doi:10.1073/pnas.0900591106
Canning BJ (2004) Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 557:543–558. doi:10.1113/jphysiol.2003.057885
Canning BJ (2006) Anatomy and neurophysiology of the cough reflex: ACCP evidence-based clinical practice guidelines. Chest 129:33S–47S. doi:10.1378/chest.129.1_suppl.33S
Canning BJ, Mori N (2011) Encoding of the cough reflex in anesthetized guinea pigs. Am J Physiol-Regul Integr Comp Physiol 300:R369–R377. doi:10.1152/ajpregu.00044.2010
Canning BJ, Mori N, Mazzone SB (2006) Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol 152:223–242. doi:10.1016/j.resp.2006.03.001
Carr MJ, Undem BJ (2003) Pharmacology of vagal afferent nerve activity in guinea pig airways. Pulm Pharmacol Ther 16:45–52. doi:10.1016/S1094-5539(02)00179-7
Carr MJ, Hunter DD, Jacoby DB, Undem BJ (2002) Expression of tachykinins in nonnociceptive vagal afferent neurons during respiratory viral infection in guinea pigs. doi:10.1164/rccm.2108065
Caterina MJ, Schumacher MA, Tominaga M et al (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824. doi:10.1038/39807
Centers for Disease Control and Prevention (2007) Infant deaths associated with cough and cold medications—two states, 2005. MMWR Morb Mortal Wkly Rep 56:1–4
Cheng W, Sun C, Zheng J (2010) Heteromerization of TRP channel subunits: extending functional diversity. Protein Cell 1:802–810. doi:10.1007/s13238-010-0108-9
Chizh BA, Sang CN (2009) Use of sensory methods for detecting target engagement in clinical trials of new analgesics. Neurotherapeutics 6:749–754. doi:10.1016/j.nurt.2009.08.005
Chou Y-L, Scarupa MD, Mori N, Canning BJ (2008) Differential effects of airway afferent nerve subtypes on cough and respiration in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 295:R1572–R1584. doi:10.1152/ajpregu.90382.2008
Choudry NB, Fuller RW (1992) Sensitivity of the cough reflex in patients with chronic cough. Eur Respir J 5:296–300, PMID: 1572441
Choudry NB, Fuller RW, Pride NB (1989) Sensitivity of the human cough reflex: effect of inflammatory mediators prostaglandin E2, bradykinin, and histamine. Am Rev Respir Dis 140:137–141, PMID: 2751160
Chuaychoo B, Hunter DD, Myers AC et al (2005) Allergen-induced substance P synthesis in large-diameter sensory neurons innervating the lungs. J Allergy Clin Immunol 116:325–331. doi:10.1016/j.jaci.2005.04.005
Clapham DE (2003) TRP channels as cellular sensors. Nature 426:517–524. doi:10.1038/nature02196
Clapham DE, Runnels LW, Strübing C (2001) The TRP ion channel family. Nat Rev Neurosci 2:387–396. doi:10.1038/35077544
Coleridge JC, Coleridge HM (1984) Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99:1–110, PMID: 6675127
Coyle MA, Keenan DB, Henderson LS et al (2005) Evaluation of an ambulatory system for the quantification of cough frequency in patients with chronic obstructive pulmonary disease. Cough 1:3. doi:10.1186/1745-9974-1-3
Crooks SW, Bayley DL, Hill SL, Stockley RA (2000) Bronchial inflammation in acute bacterial exacerbations of chronic bronchitis: the role of leukotriene B4. Eur Respir J 15:274–280. doi:10.1034/j.1399-3003.2000.15b09.x
Cullinan P (1992) Persistent cough and sputum: prevalence and clinical characteristics in south east England. Respir Med 86:143–149, PMID: 1615181
Curley FJ, Irwin RS, Pratter MR et al (1988) Cough and the common cold. Am Rev Respir Dis 138:305–311. doi:10.1164/ajrccm/138.2.305
Dai Y, Wang S, Tominaga M et al (2007) Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest 117:1979–1987. doi:10.1172/JCI30951
De Petrocellis L, Vellani V, Schiano-Moriello A et al (2008) Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther 325:1007–1015. doi:10.1124/jpet.107.134809
Decalmer SC, Webster D, Kelsall AA et al (2007) Chronic cough: how do cough reflex sensitivity and subjective assessments correlate with objective cough counts during ambulatory monitoring? Thorax 62:329–334. doi:10.1136/thx.2006.067413
Dickenson AH, Dray A (1991) Selective antagonism of capsaicin by capsazepine: evidence for a spinal receptor site in capsaicin-induced antinociception. Br J Pharmacol 104:1045–1049. doi:10.1111/j.1476-5381.1991.tb12547.x
Dicpinigaitis PV (2003) Short- and long-term reproducibility of capsaicin cough challenge testing. Pulm Pharmacol Ther 16:61–65. doi:10.1016/S1094-5539(02)00149-9
Dicpinigaitis PV (2006) Potential future therapies for the management of cough: ACCP evidence-based clinical practice guidelines. Chest 129:169S–173S. doi:10.1378/chest.129.1_suppl.248S
Dicpinigaitis PV (2007) Experimentally induced cough. Pulm Pharmacol Ther 20:319–324. doi:10.1016/j.pupt.2006.10.003
Dicpinigaitis PV, Alva RV (2005) Safety of capsaicin cough challenge testing. Chest 128:196–202, PMID: 16002935
Dicpinigaitis PV, Bhat R, Rhoton WA et al (2011) Effect of viral upper respiratory tract infection on the urge-to-cough sensation. Respir Med 105:615–618. doi:10.1016/j.rmed.2010.12.002
Dicpinigaitis PV, Morice AH, Birring SS et al (2014) Antitussive Drugs — Past, Present, and Future. Pharmacol Rev 66:468–512. doi:10.1124/pr.111.005116
Doherty MJ, Mister R, Pearson MG, Calverley PM (2000) Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax 55:643–649. doi:10.1136/thorax.55.8.643
Dubuis E, Grace M, Wortley MA et al. (2013) Harvesting, isolation, and functional assessment of primary vagal ganglia cells. Curr Protoc Pharmacol 62:Unit 12.15. doi:10.1002/0471141755.ph1215s62
Dubuis E, Wortley MA, Grace MS et al. (2014) Theophylline inhibits the cough reflex through a novel mechanism of action⋆. J Allergy Clin Immunol 1–11. doi:10.1016/j.jaci.2013.11.017
Eccles R (2006) Mechanisms of the placebo effect of sweet cough syrups. Respir Physiol Neurobiol 152:340–348. doi:10.1016/j.resp.2005.10.004
Eid SR (2011) Therapeutic targeting of TRP channels—the TR(i)P to pain relief. Curr Top Med Chem 11:2118–2130, PMID: 21671881
Ernfors P, Merlio J-P, Persson H (1992) Cells expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur J Neurosci 4:1140–1158. doi:10.1111/j.1460-9568.1992.tb00141.x
Everaerts W, Nilius B, Owsianik G (2010) The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol 103:2–17. doi:10.1016/j.pbiomolbio.2009.10.002
Fahim A, Dettmar PW, Morice AH, Hart SP (2011) Gastroesophageal reflux and idiopathic pulmonary fibrosis: a prospective study. Medicina (Kaunas) 47:200–205, PMID: 21829051
Faruqi S, Murdoch RD, Allum F, Morice AH (2014) On the definition of chronic cough and current treatment pathways: an international qualitative study. Cough 10:5. doi:10.1186/1745-9974-10-5
Fontana GA, Pantaleo T, Lavorini F et al (1999) Coughing in laryngectomized patients. Am J Respir Crit Care Med 160:1578–1584. doi:10.1164/ajrccm.160.5.9901093
Footitt J, Johnston SL (2009) Cough and viruses in airways disease: mechanisms. Pulm Pharmacol Ther 22:108–113. doi:10.1016/j.pupt.2008.12.022
Ford AC, Forman D, Moayyedi P, Morice AH (2006) Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax 61:975–979. doi:10.1136/thx.2006.060087
Fox A, Barnes P, Urban L, Dray A (1993) An in vitro study of the properties of single vagal afferents innervating guinea-pig airways. J Physiol 469:21–35. PMID: PMC1143859
Fox AJ, Urban L, Barnes PJ, Dray A (1995) Effects of capsazepine against capsaicin- and proton-evoked excitation of single airway C-fibres and vagus nerve from the guinea-pig. Neuroscience 67:741–752, PMID: 7675200
French CL (1998) Impact of chronic cough on quality of life. Arch Intern Med 158:1657–1661. PMID: 10.1001/archinte.158.15.1657
French CT, Irwin RS, Fletcher KE, Adams TM (2002) Evaluation of a cough-specific quality-of-life questionnaire. Chest 121:1123–1131, PMID: 11948042
Fuller RW, Choudry NB (1987) Increased cough reflex associated with angiotensin converting enzyme inhibitor cough. Br Med J (Clin Res Ed) 295:1025–6. PMID: PMC1248071
Fuller RW, Collier JG (1984) Sodium cromoglycate and atropine block the fall in FEV1 but not the cough induced by hypotonic mist. Thorax 39:766–770, PMID: 6437001
Gardiner PJ, Browne JL (1984) Tussive activity of inhaled PGD2 in the cat and characterisation of the receptor(s) involved. Prostaglandins Leukot Med 14:153–159, PMID: 6328541
Gatti R, Andre E, Amadesi S et al (2006) Protease-activated receptor-2 activation exaggerates TRPV1-mediated cough in guinea pigs. J Appl Physiol 101:506–511. doi:10.1152/japplphysiol.01558.2005
Gavva NR, Bannon AW, Hovland DN et al (2007) Repeated administration of vanilloid receptor TRPV1 antagonists attenuates hyperthermia elicited by TRPV1 blockade. J Pharmacol Exp Ther 323:128–137. doi:10.1124/jpet.107.125674
Gavva NR, Treanor JJS, Garami A et al (2008) Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 136:202–210. doi:10.1016/j.pain.2008.01.024
Geppetti P, Materazzi S, Nicoletti P (2006) The transient receptor potential vanilloid 1: role in airway inflammation and disease. Eur J Pharmacol 533:207–214, PMID: 16464449
Gompertz S, O’Brien C, Bayley DL, Hill SL, Stockley RA (2001) Changes in bronchial inflammation during acute exacerbations of chronic bronchitis. Eur Respir J 17:1112–1119, PMID: 11491152
Grace MS, Belvisi MG (2011) TRPA1 receptors in cough. Pulm Pharmacol Ther 24:286–288, PMID: 21074632
Grace M, Birrell MA, Dubuis E et al (2012) Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax 67:891–900. doi:10.1136/thoraxjnl-2011-201443
Grace MS, Dubuis E, Birrell MA, Belvisi MG (2013) Pre-clinical studies in cough research: role of transient receptor potential (TRP) channels. Pulm Pharmacol Ther 26:498–507. doi:10.1016/j.pupt.2013.02.007
Grace MS, Lieu T, Darby B et al (2014a) The tyrosine kinase inhibitor bafetinib inhibits PAR2-induced activation of TRPV4 in vitro and pain in vivo. Br J Pharmacol. doi:10.1111/bph.12750
Grace MS, Baxter M, Dubuis E, Birrell MA, Belvisi MG (2014b) Transient receptor potential (TRP) channels in the airway: role in airway disease. Br J Pharmacol 171:2593–2607. doi:10.1111/bph.12538
Grant AD, Cottrell GS, Amadesi S et al (2007) Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol 578:715–733. doi:10.1113/jphysiol.2006.121111
Groneberg DA (2004) Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med 170:1276–1280. doi:10.1164/rccm.200402-174OC
Gunthorpe MJ, Chizh BA (2009) Clinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathway. Drug Discov Today 14:56–67. doi:10.1016/j.drudis.2008.11.005
Hadley SH, Bahia PK, Taylor-Clark TE (2014) Sensory nerve terminal mitochondrial dysfunction induces hyperexcitability in airway nociceptors via protein kinase C. Mol Pharmacol 85:839–848. doi:10.1124/mol.113.091272
Hanácek J, Davies A, Widdicombe JG (1984) Influence of lung stretch receptors on the cough reflex in rabbits. Respiration 45:161–168, PMID: 6463386
Haque RA, Usmani OS, Barnes PJ (2005) Chronic idiopathic cough: a discrete clinical entity. Chest 127:1710–1713. doi:10.1378/chest.127.5.1710
Hilton ECY, Baverel PG, Woodcock A et al (2013) Pharmacodynamic modeling of cough responses to capsaicin inhalation calls into question the utility of the C5 end point. J Allergy Clin Immunol. doi:10.1016/j.jaci.2013.04.042
Hinman A, Chuang H-H, Bautista DM, Julius D (2006) TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A 103:19564–19568. doi:10.1073/pnas.0609598103
Hondoh A, Ishida Y, Ugawa S et al (2010) Distinct expression of cold receptors (TRPM8 and TRPA1) in the rat nodose-petrosal ganglion complex. Brain Res 1319:60–69. doi:10.1016/j.brainres.2010.01.016
Hu Y, Liu Z, Yu X et al (2014) Increased acid responsiveness in vagal sensory neurons in a guinea pig model of eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. doi:10.1152/ajpgi.00097.2014
Hunter DD, Undem BJ (1999) Identification and substance P content of vagal afferent neurons innervating the epithelium of the guinea pig trachea. Am J Respir Crit Care Med 159:1943–1948. doi:10.1164/ajrccm.159.6.9808078
Hwang SW, Cho H, Kwak J et al (2000) Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A 97:6155–6160. doi:10.1073/pnas.97.11.6155
Ichikawa H, Sugimoto T (2004) The co-expression of P2X3 receptor with VR1 and VRL-1 in the rat trigeminal ganglion. Brain Res 998:130–135. doi:10.1016/j.brainres.2003.11.019
Irwin RS, Boulet LP, Cloutier MM et al (1998) Managing cough as a defense mechanism and as a symptom. A consensus panel report of the American College of Chest Physicians. Chest 114:133S–181S. doi:10.1378/chest/114.2_Supplement 133S
Jang Y, Lee Y, Kim SM et al (2012) Quantitative analysis of TRP channel genes in mouse organs. Arch Pharm Res 35:1823–1830. doi:10.1007/s12272-012-1016-8
Janson C, Chinn S, Jarvis D, Burney P (2001) Determinants of cough in young adults participating in the European Community Respiratory Health Survey. Eur Respir J 18:647–654, PMID: 11716169
Jaquemar D, Schenker T, Trueb B (1999) An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem 274:7325–7333, PMID: 10066796
Jia Y, McLeod RL, Wang X et al (2002) Anandamide induces cough in conscious guinea-pigs through VR1 receptors. Br J Pharmacol 137:831–836, PMID: 12411414
Jia Y, Wang X, Varty L et al (2004) Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 287:L272–L278. doi:10.1152/ajplung.00393.2003
Jordt SE, Tominaga M, Julius D (2000) Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U S A 97:8134–8139. doi:10.1073/pnas.100129497
Jordt S, Bautista DM, Chuang H et al (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. doi:10.1038/nature02237.1
Kagaya M, Lamb J, Robbins J et al (2002) Characterization of the anandamide induced depolarization of guinea-pig isolated vagus nerve. Br J Pharmacol 137:39–48. doi:10.1038/sj.bjp.0704840
Kajekar R, Proud D, Myers AC et al (1999) Characterization of vagal afferent subtypes stimulated by bradykinin in guinea pig trachea. J Pharmacol Exp Ther 289:682–687, PMID: 10215640
Kamei J, Iwamoto Y, Suzuki T et al (1993) Antitussive effects of naltrindole, a selective delta-opioid receptor antagonist, in mice and rats. Eur J Pharmacol 249:161–165, PMID: 10640321
Kaneko Y, Szallasi A (2014) Transient receptor potential (TRP) channels: a clinical perspective. Br J Pharmacol 171:2474–2507. doi:10.1111/bph.12414
Karashima Y, Damann N, Prenen J et al. (2007) Bimodal action of menthol on the transient receptor potential channel TRPA1. J. Neurosci. (37): 9874-9884; doi: 10.1523/JNEUROSCI.2221-07.2007
Karlsson JA, Fuller RW (1999) Pharmacological regulation of the cough reflex–from experimental models to antitussive effects in Man. Pulm Pharmacol Ther 12:215–228. doi:10.1006/pupt.1999.0207
Karlsson JA, Hansson L, Wollmer P, Dahlbäck M (1991a) Regional sensitivity of the respiratory tract to stimuli causing cough and reflex bronchoconstriction. Respir Med 85 Suppl A:47–50. PMID: 2034835
Karlsson JA, Zackrisson C, Sjölin C, Forsberg K (1991b) Cigarette smoke-induced changes in guinea-pig airway responsiveness to histamine and citric acid. Acta Physiol Scand 142:119–125. doi:10.1111/j.1748-1716.1991.tb09136.x
Kawakami Y, Uchiyama K, Irie T, Murao M (1973) Evaluation of aerosols of prostaglandins E1 and E2 as bronchodilators. Eur J Clin Pharmacol 6:127–132, PMID: 4588852
Keatings VM, Collins PD, Scott DM, Barnes PJ (1996) Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med 153:530–534, PMID: 8564092
Keh SM, Facer P, Yehia A et al (2011) The menthol and cold sensation receptor TRPM8 in normal human nasal mucosa and rhinitis. Rhinology 49:453–457. doi:10.4193/Rhino11.089
Kelsall A, Houghton LA, Jones H et al (2011) A novel approach to studying the relationship between subjective and objective measures of cough. Chest 139:569–575. doi:10.1378/chest. 10-0438
Key AL, Holt K, Hamilton A et al (2010) Objective cough frequency in idiopathic pulmonary fibrosis. Cough 6:4. doi:10.1186/1745-9974-6-4
Khalid S, Murdoch R, Newlands A et al (2014) Transient receptor potential vanilloid 1 (TRPV1) antagonism in patients with refractory chronic cough: a double-blind randomized controlled trial. J Allergy Clin Immunol. doi:10.1016/j.jaci.2014.01.038
Kogan MD, Pappas G, Yu SM, Kotelchuck M (1994) Over-the-counter medication use among US preschool-age children. J Am Med Assoc 272:1025–1030, PMID: 8089884
Kollarik M, Undem BJ (2002) Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol 543:591–600. doi:10.1113/jphysiol.2002.022848
Kollarik M, Undem BJ (2004) Activation of bronchopulmonary vagal afferent nerves with bradykinin, acid and vanilloid receptor agonists in wild-type and TRPV1-/- mice. J Physiol 555:115–123, PMID: 14634201
Koskela HO, Kontra KM, Purokivi MK, Randell JT (2005) Interpretation of cough provoked by airway challenges. Chest 128:3329–3335, PMID: 16304280
Krarup AL, Ny L, Astrand M et al (2011) Randomised clinical trial: the efficacy of a transient receptor potential vanilloid 1 antagonist AZD1386 in human oesophageal pain. Aliment Pharmacol Ther 33:1113–1122. doi:10.1111/j.1365-2036.2011.04629.x
Kunert-Keil C, Bisping F, Krüger J, Brinkmeier H (2006). Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics 7:159.PMID: 16787531
Kwon M, Baek SH, Park C-K et al (2014) Single-cell RT-PCR and immunocytochemical detection of mechanosensitive transient receptor potential channels in acutely isolated rat odontoblasts. Arch Oral Biol 59:1266–1271. doi:10.1016/j.archoralbio.2014.07.016
Kwong K, Lee L-Y (2005) Prostaglandin E2 potentiates a TTX-resistant sodium current in rat capsaicin-sensitive vagal pulmonary sensory neurones. J Physiol 564:437–450. doi:10.1113/jphysiol.2004.078725
Lalloo UG, Fox AJ, Belvisi MG et al (1995) Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J Appl Physiol 79:1082–1087, PMID: 8567546
Lalloo UG, Barnes PJ, Chung KF (1996) Pathophysiology and clinical presentations of cough. J Allergy Clin Immunol 98:S91–S97. doi:10.1016/S0091-6749(96)70022-2
Lashinger ESR, Steiginga MS, Hieble JP et al (2008) AMTB, a TRPM8 channel blocker: evidence in rats for activity in overactive bladder and painful bladder syndrome. Am J Physiol Renal Physiol 295:F803–F810. doi:10.1152/ajprenal.90269.2008
Latorre R, Brauchi S, Orta G et al (2007) ThermoTRP channels as modular proteins with allosteric gating. Cell Calcium 42:427–438, PMID: 17499848
Latorre R, Zaelzer C, Brauchi S (2009) Structure-functional intimacies of transient receptor potential channels. Q Rev Biophys 42:201–246. doi:10.1017/S0033583509990072
Laude EA, Higgins KS, Morice AH (1993) A comparative study of the effects of citric acid, capsaicin and resiniferatoxin on the cough challenge in guinea-pig and man. Pulm Pharmacol 6:171–175, PMID: 8219571
Laude EA, Morice AH, Grattan TJ (1994) The antitussive effects of menthol, camphor and cineole in conscious guinea-pigs. Pulm Pharmacol 7:179–184. doi:10.1006/pulp.1994.1021
Lee M-G, Undem BJ, Brown C, Carr MJ (2006) Effect of nociceptin in acid-evoked cough and airway sensory nerve activation in guinea pigs. Am J Respir Crit Care Med 173:271–275, PMID: 16239621
Lee L-YY, Burki NK, Gerhardstein DC et al (2007) Airway irritation and cough evoked by inhaled cigarette smoke: role of neuronal nicotinic acetylcholine receptors. Pulm Pharmacol Ther 20:355–364, PMID: 17137814
Lewis CA, Ambrose C, Banner K et al (2007) Animal models of cough: literature review and presentation of a novel cigarette smoke-enhanced cough model in the guinea-pig. Pulm Pharmacol Ther 20:325–333. doi:10.1016/j.pupt.2006.12.001
Liedtke W, Friedman JM (2003) Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci U S A 100:13698–13703. doi:10.1073/pnas.1735416100
Liedtke W, Choe Y, Martí-Renom MA et al (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103:525–535. doi:10.1016/S0092-8674(00)00143-4
Lieu TM, Myers AC, Meeker S, Undem BJ (2012) TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am J Physiol Lung Cell Mol Physiol 302:L941–L948. doi:10.1152/ajplung.00366.2011
Lin YS, Hsu C-C, Bien M-Y et al (2010) Activations of TRPA1 and P2X receptors are important in ROS-mediated stimulation of capsaicin-sensitive lung vagal afferents by cigarette smoke in rats. J Appl Physiol 108:1293–1303. doi:10.1152/japplphysiol.01048.2009
Lowry RH, Wood AM, Higenbottam TW (1988) Effects of pH and osmolarity on aerosol-induced cough in normal volunteers. Clin Sci 74:373–376, PMID: 3356109
Lundberg JM, Franco-Cereceda A, Hua X et al (1985) Co-existence of substance P and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Eur J Pharmacol 108:315–319, PMID: 1854545
Ma S, G G, Ak V-E et al. (2008) Menthol derivative WS-12 selectively activates transient receptor potential melastatin-8 (TRPM8) ion channels. Pak J Pharm Sci 21:370–8. PMID: 18930858
Macpherson LJ, Geierstanger BH, Viswanath V et al (2005) The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol 15:929–934. doi:10.1016/j.cub.2005.04.018
Madison JM, Irwin RS (2010) Cough: a worldwide problem. Otolaryngol Clin North Am 43:1–13, vii. doi: 10.1016/j.otc.2009.11.001
Maher S A, Belvisi MG (2010) Prostanoids and the cough reflex. Lung 188 Suppl :S9–12. doi:10.1007/s00408-009-9190-2
Maher SA, Birrell MA, Belvisi MG (2009) Prostaglandin E2 mediates cough via the EP3 receptor: implications for future disease therapy. Am J Respir Crit Care Med 180:923–928. doi:10.1164/rccm.200903-0388OC
Maher MP, Bhattacharya A, Ao H et al (2011) Characterization of 2-(2,6-dichloro-benzyl)-thiazolo[5,4-d]pyrimidin-7-yl]-(4-trifluoromethyl-phenyl)-amine (JNJ-39729209) as a novel TRPV1 antagonist. Eur J Pharmacol 663:40–50. doi:10.1016/j.ejphar.2011.05.001
Materazzi S, Nassini R, Gatti R et al. (2009) Cough sensors. II. Transient receptor potential membrane receptors on cough sensors. Handb Exp Pharmacol 49–61. doi: 10.1007/978-3-540-79842-2_3
Mazzone SB (2004) Sensory regulation of the cough reflex. Pulm Pharmacol Ther 17:361–368, PMID: 15564077
McAlexander MA, Luttmann MA, Hunsberger GE, Undem BJ (2014) Transient receptor potential vanilloid 4 activation constricts the human bronchus via the release of cysteinyl leukotrienes. J Pharmacol Exp Ther 349:118–125. doi:10.1124/jpet.113.210203
McGarvey LP, Heaney LG, Lawson JT et al (1998) Evaluation and outcome of patients with chronic non-productive cough using a comprehensive diagnostic protocol. Thorax 53:738–743. doi:10.1136/thx.53.9.738
McGarvey LPA, Polley L, MacMahon J (2007) Common causes and current guidelines. Chron Respir Dis 4:215–223. doi:10.1177/1479972307084447
McGuinness K, Morice A, Woodcock A, Smith J (2008) The Leicester Cough Monitor: a semi-automated, semi-validated cough detection system? Eur Respir J 32:529–530. doi:10.1183/09031936.00052008, author reply 530–1
McGuinness K, Holt K, Dockry R, Smith J (2012) P159 Validation of the VitaloJAK 24 hour ambulatory cough monitor. Thorax 67:A131. doi:10.1136/thoraxjnl-2012-202678.220
McKemy DD, Neuhausser WM, Julius D (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416:52–58. doi:10.1038/nature719
McLeod RL, Fernandez X, Correll CC et al (2006) TRPV1 antagonists attenuate antigen-provoked cough in ovalbumin sensitized guinea pigs. Cough 2:10. doi:10.1186/1745-9974-2-10
McNamara CR, Mandel-Brehm J, Bautista DM et al (2007) TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci 104:13525–13530. doi:10.1073/pnas.0705924104
Montell C, Rubin GM (1989) Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 2:1313–1323. doi:10.1016/0896-6273(89)90069-X
Moran MM, McAlexander MA, Bíró T, Szallasi A (2011) Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov 10:601–620. doi:10.1038/nrd3456
Morice AH, Marshall AE, Higgins KS, Grattan TJ (1994) Effect of inhaled menthol on citric acid induced cough in normal subjects. Thorax 49:1024–1026. doi:10.1136/thx.49.10.1024
Morice AH, Kastelik JA, Thompson R (2001) Cough challenge in the assessment of cough reflex. Br J Clin Pharmacol 52:365–375. doi:10.1046/j.0306-5251.2001.01475.x
Morice AH, Fontana GA, Belvisi MG et al (2007a) ERS guidelines on the assessment of cough. Eur Respir J 29:1256–1276. doi:10.1183/09031936.00101006
Morice AH, Menon MS, Mulrennan SA et al (2007b) Opiate therapy in chronic cough. Am J Respir Crit Care Med 175:312–315. doi:10.1164/rccm.200607-892OC
Moriyama T, Higashi T, Togashi K et al (2005) Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain 1:3. doi:10.1186/1744-8069-1-3
Mortola J, Sant’Ambrogio G, Clement MG (1975) Localization of irritant receptors in the airways of the dog. Respir Physiol 24:107–114, PMID: 4772408
Mukhopadhyay I, Gomes P, Aranake S, Shetty M, Karnik P, Damle M et al (2011) Expression of functional TRPA1 receptor on human lung fibroblast and epithelial cells. J Recept Signal Transduct Res 31:350–358. doi:10.3109/10799893.2011.602413
Mukhopadhyay I, Kulkarni A, Aranake S et al (2014) Transient receptor potential ankyrin 1 receptor activation in vitro and in vivo by pro-tussive agents: GRC 17536 as a promising anti-tussive therapeutic. PLoS One 9:e97005. doi:10.1371/journal.pone.0097005
Myers AC, Kajekar R, Undem BJ (2002) Allergic inflammation-induced neuropeptide production in rapidly adapting afferent nerves in guinea pig airways. Am J Physiol Lung Cell Mol Physiol 282:L775–L781. doi:10.1152/ajplung.00353.2001
Nasra J, Belvisi MG (2009) Modulation of sensory nerve function and the cough reflex: understanding disease pathogenesis. Pharmacol Ther 124:354–375. doi:10.1016/j.pharmthera.2009.09.006
Nassenstein C, Kwong K, Taylor-Clark T et al (2008) Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol 586:1595–1604. doi:10.1113/jphysiol.2007.148379
Ni D, Gu Q, Hu H-Z et al (2006) Thermal sensitivity of isolated vagal pulmonary sensory neurons: role of transient receptor potential vanilloid receptors. Am J Physiol Regul Integr Comp Physiol 291:R541–R550. doi:10.1152/ajpregu.00016.2006
Nieto L, de Diego A, Perpiñá M et al (2003) Cough reflex testing with inhaled capsaicin in the study of chronic cough. Respir Med 97:393–400, PMID: 12693800
Nilius B (2007) TRP channels in disease. Biochim Biophys Acta 1772:805–812. doi:10.1016/j.bbadis.2007.02.002
Nilius B, Szallasi A (2014) Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev 676–814. doi: 10.1124/pr.113.008268
Patel HJ, Birrell MA, Crispino N et al (2003) Inhibition of guinea-pig and human sensory nerve activity and the cough reflex in guinea-pigs by cannabinoid (CB2) receptor activation. Br J Pharmacol 140:261–268. doi:10.1038/sj.bjp.0705435
Patwardhan AM, Akopian AN, Ruparel NB et al (2010) Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest 120:1617–1626. doi:10.1172/JCI41678
Peier AM, Moqrich A, Hergarden AC et al (2002) A TRP channel that senses cold stimuli and menthol. Cell 108:705–715, PMID: 11893340
Picazo-Juárez G, Romero-Suárez S, Nieto-Posadas A et al (2011) Identification of a binding motif in the S5 helix that confers cholesterol sensitivity to the TRPV1 ion channel. J Biol Chem 286:24966–24976. doi:10.1074/jbc.M111.237537
Plevkova J, Kollarik M, Poliacek I et al (2013) The role of trigeminal nasal TRPM8-expressing afferent neurons in the antitussive effects of menthol. J Appl Physiol 115:268–274. doi:10.1152/japplphysiol.01144.2012
Polley L, Yaman N, Heaney L et al (2008) Impact of cough across different chronic respiratory diseases: comparison of two cough-specific health-related quality of life questionnaires. Chest 134:295–302. doi:10.1378/chest. 07-0141
Poole DP, Amadesi S, Veldhuis NA et al (2013) Protease-activated receptor 2 (PAR2) protein and transient receptor potential vanilloid 4 (TRPV4) protein coupling is required for sustained inflammatory signaling. J Biol Chem 288:5790–5802. doi:10.1074/jbc.M112.438184
Preti D, Szallasi A, Patacchini R (2012) TRP channels as therapeutic targets in airway disorders: a patent review. Expert Opin Ther Pat 22:663–695. doi:10.1517/13543776.2012.696099
Prudon B, Birring SS, Vara DD et al (2005) Cough and glottic-stop reflex sensitivity in health and disease. Chest 127:550–557. doi:10.1378/chest.127.2.550
Radresa O, Paré M, Albert JS (2013) Multiple roles of transient receptor potential (TRP) channels in inflammatory conditions and current status of drug development. Curr Top Med Chem 13:367–385. doi:10.2174/1568026611313030012
Ramsey IS, Delling M, Clapham DE (2006) An introduction to TRP channels. Annu Rev Physiol 68:619–647. doi:10.1146/annurev.physiol.68.040204.100431
Riccio M, Kummer W, Biglari B et al. (1996) Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol 496 (Pt 2:521–530. PMID: 8910234
Round P, Priestley A, Robinson J (2011) An investigation of the safety and pharmacokinetics of the novel TRPV1 antagonist XEN-D0501 in healthy subjects. Br J Clin Pharmacol 72:921–931. doi:10.1111/j.1365-2125.2011.04040.x
Sant’Ambrogio G, Remmers JE, de Groot WJ et al (1978) Localization of rapidly adapting receptors in the trachea and main stem bronchus of the dog. Respir Physiol 33:359–366. doi:10.1016/0034-5687(78)90062-2
Sawada Y, Hosokawa H, Matsumura K, Kobayashi S (2008) Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur J Neurosci 27:1131–1142. doi:10.1111/j.1460-9568.2008.06093.x
Schappert SM, Burt CW (2006) Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 2001-02. Vital Health Stat 13:1–66
Schappert SM, Rechtsteiner EA (2011) Ambulatory medical care utilization estimates for 2007. Vital Health Stat 13:1–38, PMID: 21614897
Schelegle ES (2003) Functional morphology and physiology of slowly adapting pulmonary stretch receptors. Anat Rec A: Discov Mol Cell Evol Biol 270:11–16. doi:10.1002/ar.a.10004
Schelegle ES, Green JF (2001) An overview of the anatomy and physiology of slowly adapting pulmonary stretch receptors. Respir Physiol 125:17–31, PMID: 11240150
Schoeffel RE, Anderson SD, Altounyan REC CLINICAL (1981) Bronchial hyperreactivity in response to inhalation of ultrasonically nebulised solutions of distilled water and saline. BMJ 283:1285–1287, PMID: 1507674
Schroeder K, Fahey T (2002) Systematic review of randomised controlled trials of over the counter cough medicines for acute cough in adults. BMJ 324:1–6. PMCID: PMC65295
Seabrook GR, Sutton KG, Jarolimek W et al (2002) Functional properties of the high-affinity TRPV1 (VR1) vanilloid receptor antagonist (4-hydroxy-5-iodo-3-methoxyphenylacetate ester) iodo-resiniferatoxin. J Pharmacol Exp Ther 303:1052–1060. doi:10.1124/jpet.102.040394
Sesoko S, Kaneko Y (1985) Cough associated with the use of captopril. Arch Intern Med 145:1524, PMID: 3896184
Shapiro D, Deering-Rice CE, Romero EG et al. (2013) Activation of transient receptor potential ankyrin-1 (TRPA1) in lung cells by wood smoke particulate material. Chem. Res. Toxicol. 750-758. doi: 10.1021/tx400024h
Smart D, Gunthorpe MJ, Jerman JC et al (2000) The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br J Pharmacol 129:227–230. doi:10.1038/sj.bjp.0703050
Smit LA, Kogevinas M, Antó JM et al (2012) Transient receptor potential genes, smoking, occupational exposures and cough in adults. Respir Res 13:26. doi:10.1186/1465-9921-13-26
Smith J (2010) Monitoring chronic cough: current and future techniques. Expert Rev Respir Med 4:673–683. doi:10.1586/ers.10.63
Smith J, Woodcock A (2008) New developments in the objective assessment of cough. Lung 186 Suppl :S48–54. PMID: 18066694
Smith J, Owen E, Earis J, Woodcock A (2006a) Effect of codeine on objective measurement of cough in chronic obstructive pulmonary disease. J Allergy Clin Immunol 117:831–835. doi:10.1016/j.jaci.2005.09.055
Smith PL, Maloney KN, Pothen RG et al (2006b) Bisandrographolide from Andrographis paniculata activates TRPV4 channels. J Biol Chem 281:29897–29904. doi:10.1074/jbc.M605394200
Smith JA, Hilton ECY, Saulsberry L, Canning BJ (2012) Antitussive effects of memantine in guinea pigs. Chest 141:996–1002. doi:10.1378/chest. 11-0554
Standring S (2005) Gray’s anatomy: the anatomical basis of clinical practice, 39th Edition. 784–786.
Stokes A, Wakano C, Koblam-Huberson M et al (2006) TRPA1 is a substrate for deubiquitination by the tumor suppressor CYLD. Cell Signal 18:1584–1594, PMID: 16500080
Story GM, Peier AM, Reeve AJ et al (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112:819–829. doi:10.1016/S0092-8674(03)00158-2
Strotmann R, Harteneck C, Nunnenmacher K et al (2000) OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2:695–702. doi:10.1038/35036318
Sumner H, Woodcock A Kolsum U et al. (2013) Predictors of objective cough frequency in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1–32. doi:10.1164/rccm.201211-2000OC
Suzuki M, Mizuno A, Kodaira K, Imai M (2003) Impaired pressure sensation in mice lacking TRPV4. J Biol Chem 278:22664–22668. doi:10.1074/jbc.M302561200
Swanson DM, Dubin AE, Shah C et al (2005) Identification and biological evaluation of 4-(3-trifluoromethylpyridin-2-yl)piperazine-1-carboxylic acid (5-trifluoromethylpyridin-2-yl)amide, a high affinity TRPV1 (VR1) vanilloid receptor antagonist. J Med Chem 48:1857–1872. doi:10.1021/jm0495071
Szallasi A, Blumberg PM (1990) Specific binding of resiniferatoxin, an ultrapotent capsaicin analog, by dorsal root ganglion membranes. Brain Res 524:106–111
Takahashi N, Mizuno Y, Kozai D et al (2008) Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels (Austin) 2:287–298, PMID: 18769139
Takahashi N, Kuwaki T, Kiyonaka S et al (2011) TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol 7:701–711. doi:10.1038/nchembio.640
Takemura M, Quarcoo D, Niimi A et al (2008) Is TRPV1 a useful target in respiratory diseases? Pulm Pharmacol Ther 21:833–839. doi:10.1016/j.pupt.2008.09.005
Tatar M, Sant’Ambrogio G, Sant’Ambrogio FB (1994) Laryngeal and tracheobronchial cough in anesthetized dogs. J Appl Physiol 76:2672–2679, PMID: 7928899
Taylor-Clark TE, Undem BJ (2010) Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol 588:423–433. doi:10.1113/jphysiol.2009.183301
Taylor-Clark TE, McAlexander MA, Nassenstein C et al (2008) Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol 586:3447–3459. doi:10.1113/jphysiol.2008.153585
Taylor-Clark TE, Nassenstein C, McAlexander MA, Undem BJ (2009) TRPA1: a potential target for anti-tussive therapy. Pulm Pharmacol Ther 22:71–74. doi:10.1016/j.pupt.2008.12.019
Terada Y, Fujimura M, Nishimura S et al. (2013) Contribution of TRPA1 as a downstream signal of proteinase-activated receptor-2 to pancreatic pain. J Pharmacol Sci 123:284–7.PMID:24162021
Ternesten-Hasséus E, Johansson K, Löwhagen O, Millqvist E (2006) Inhalation method determines outcome of capsaicin inhalation in patients with chronic cough due to sensory hyperreactivity. Pulm Pharmacol Ther 19:172–178. doi:10.1016/j.pupt.2005.04.010
Thorneloe KS, Sulpizio AC, Lin Z et al. (2008) N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: part I. J Pharmacol Exp Ther 326:432–442. doi:10.1124/jpet.108.139295
Thorneloe KS, Cheung M, Bao W et al. (2012) An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med 4:159ra148. doi: 10.1126/scitranslmed.3004276
Trevisani M, Milan A, Gatti R et al (2004) Antitussive activity of iodo-resiniferatoxin in guinea pigs. Thorax 59:769–772. doi:10.1136/thx.2003.012930
Trevisani M, Siemens J, Materazzi S et al. (2007) 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. 104: 13519-13524. doi:10.1073/pnas.0705923104
Undem BJ (2004) Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol 556:905–917, PMID: 14978204
Undem BJ, Carr MJ, Kollarik M (2002) Physiology and plasticity of putative cough fibres in the guinea pig. Pulm Pharmacol Ther 15:193–198. doi:10.1006/pupt.2002.0362
Usmani OS, Belvisi MG, Patel HJ et al (2005) Theobromine inhibits sensory nerve activation and cough. FASEB J 19:231–233. doi:10.1096/fj.04-1990fje
Valero ML, Mello de Queiroz F, Stühmer W et al (2012) TRPM8 ion channels differentially modulate proliferation and cell cycle distribution of normal and cancer prostate cells. PLoS One 7:e51825. doi:10.1371/journal.pone.0051825
Vassilev ZP, Chu AF, Ruck B et al (2009) Adverse reactions to over-the-counter cough and cold products among children: the cases managed out of hospitals. J Clin Pharm Ther 34:313–318. doi:10.1111/j.1365-2710.2008.01010.x
Vincent F, Acevedo A, Nguyen MT et al (2009) Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun 389:490–494. doi:10.1016/j.bbrc.2009.09.007
Vizel E, Yigla M, Goryachev Y et al (2010) Validation of an ambulatory cough detection and counting application using voluntary cough under different conditions. Cough 6:3. doi:10.1186/1745-9974-6-3
Volpi G, Facchinetti F, Moretto N et al (2011) Cigarette smoke and α, β-unsaturated aldehydes elicit VEGF release through the p38 MAPK pathway in human airway smooth muscle cells and lung fibroblasts. Br J Pharmacol 163:649–661. doi:10.1111/j.1476-5381.2011.01253.x
Vriens J, Nilius B, Vennekens R (2008) Herbal compounds and toxins modulating TRP channels. Curr Neuropharmacol 6:79–96. doi:10.2174/157015908783769644
Watanabe H, Davis JB, Smart D et al (2002) Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 277:13569–13577. doi:10.1074/jbc.M200062200
Watanabe H, Vriens J, Prenen J, Droogmans G (2003) Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. 424:4–8. doi:10.1038/nature01844.1.
Widdicombe JG (1995) Neurophysiology of the cough reflex. Eur Respir J 8:1193–1202, PMID: 7589405
Widdicombe J (2003) Functional morphology and physiology of pulmonary rapidly adapting receptors (RARs). Anat Rec A: Discov Mol Cell Evol Biol 270:2–10, PMID: 12494484
Widdicombe JG, Undem BJ (2002) Summary: central nervous pharmacology of cough. Pulm Pharmacol Ther 15:251–252, PMID: 12099773
Willis DN, Liu B, Ha MA et al (2011) Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J 25:4434–4444. doi:10.1096/fj.11-188383
Wise PM, Breslin PAS, Dalton P (2012) Sweet taste and menthol increase cough reflex thresholds. Pulm Pharmacol Ther 25:236–241. doi:10.1016/j.pupt.2012.03.005
Wong CH, Matai R, Morice AH (1999) Cough induced by low pH. Respir Med 93:58–61. doi:10.1016/S0954-6111(99)90078-1
Wortley MA, Grace MS, Dubuis ED et al. (2011) Cough and vagal sensory afferent responses to PGE2 are altered in a guinea pig cigarette smoke exposure model and in human smokers and COPD patients compared to normal volunteers. Proc. Br. Pharmacol. Soc. http://www.pa2online.org/abstracts/1vol9issue3abst008p.pdf
Wortley MA, Birrell MA, Maher SA et al. (2014) Profiling of Xen-D0501, a novel TRPV1 antagonist, in pre-clinical models of cough. AJRCCM A4979
Xing H, Ling JX, Chen M et al (2008) TRPM8 mechanism of autonomic nerve response to cold in respiratory airway. Mol Pain 4:22. doi:10.1186/1744-8069-4-22
Young EC, Smith JA (2011) Pharmacologic therapy for cough. Curr Opin Pharmacol 11:224–230. doi:10.1016/j.coph.2011.06.003
Yousaf N, Monteiro W, Matos S, Birring SS, Pavord ID (2013) Cough frequency in health and disease. Eur Respir J 41:241–243. doi:10.1183/09031936.00089312
Yu S (2005) Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J Physiol 563:831–842. doi:10.1113/jphysiol.2004.079574
Zhou Y, Sun B, Li Q et al (2011) Sensitivity of bronchopulmonary receptors to cold and heat mediated by transient receptor potential cation channel subtypes in an ex vivo rat lung preparation. Respir Physiol Neurobiol 177:327–332. doi:10.1016/j.resp.2011.05.011
Zhu G, Gulsvik A, Bakke P et al (2009) Association of TRPV4 gene polymorphisms with chronic obstructive pulmonary disease. Hum Mol Genet 18:2053–2062. doi:10.1093/hmg/ddp111
Zygmunt PM, Petersson J, Andersson DA et al (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400:452–457. doi:10.1038/22761
Acknowledgments
SJB was supported by a studentship from the National Heart and Lung Institute (NHLI, UK). JAS was funded by a Medical Research Council (MRC) Clinician Scientist Award (G0701918). MAB and MGB were funded by Imperial College and a project grant from the Medical Research Council (MRC, UK) (MR/K020293/1). We would like to thank Dr. Michael Wortley, Dr. Eric Dubuis and Dr. John Adcock for their help in preparing the figures in this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonvini, S.J., Birrell, M.A., Smith, J.A. et al. Targeting TRP channels for chronic cough: from bench to bedside. Naunyn-Schmiedeberg's Arch Pharmacol 388, 401–420 (2015). https://doi.org/10.1007/s00210-014-1082-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-014-1082-1