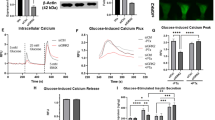
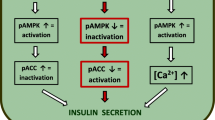
Abstract
In the majority of cell types, including the islet β-cell, transduction of extracellular signals involves ligand binding to a receptor, often followed by the activation G proteins and their effector modules. The islet β-cell is unusual in that glucose lacks an extracellular receptor. Instead, events consequent to glucose metabolism promote insulin secretion via the generation of diffusible second messengers and mobilization of calcium. A selective increase in intracellular calcium has been shown to regulate the phosphorylation status key islet proteins thereby facilitating insulin secretion. In addition to classical protein kinases [e.g., protein kinases A and C], recent studies from our laboratory have focused on the expression and function of various forms of NDPK/nm23-like histidine kinases in clonal β-cells, normal rodent, and human islets. Further, we recently reported localization of a cytosolic protein histidine phosphatase [PHP] in INS 832/13 cells, normal rat islets, and human islets. siRNA-mediated knock down of nm23-H1 and PHP in insulin-secreting INS 832/13 cells significantly attenuated glucose-induced insulin secretion. We also observed significant alterations in the expression and function of nm23-H1/PHP in β-cells chronically exposed to elevated levels of glucose and saturated fatty acids, such as palmitate (i.e., glucolipotoxicity). Similar changes were also noted in islets from the Goto-Kakizaki and Zucker Diabetic Fatty rats, two known models for type 2 diabetes. It is concluded that protein histidine phosphorylation–dephosphorylation cycles play novel regulatory roles in G protein-mediated physiological insulin secretion and that abnormalities in this signaling axis lead to impaired insulin secretion in glucolipotoxicity and type 2 diabetes.
Similar content being viewed by others
Notes
In siRNA-mediated knock down of nm23-H1 studies, we used a pool of three duplexes. The sequences for those duplexes are duplex A: sense, GGAAAGAAGUGAUCACAAAtt and antisense, UUUGUGAUCACUUCUUUCCtt; duplex B: sense, GAACAAUUCUCCAACCUAUtt and antisense, AUAGGUUGGAGAAUUGUUCtt; and duplex C: sense, GUAGCUAAUCUCUU-GUGUUtt and antisense, AACACAAGAGAU-UAGCUACtt [all sequences are provided in 5′ → 3′ orientation; purchased from Santa Cruz Biotechnology, Santa Cruz, CA].
Abbreviations
- ACL:
-
ATP-citrate lyase
- CER:
-
Ceramide
- GSIS:
-
Glucose-stimulated insulin secretion
- GK rat:
-
Goto-Kakizaki rat
- H4-HK:
-
Histone 4 phosphorylating histidine kinase
- IMPDH:
-
Inosine monophosphate dehydrogenase
- MPA:
-
Mycophenolic acid
- NDPK:
-
Nucleoside diphosphate kinase
- Nox:
-
Phagocyte-like NADPH oxidase
- PHP:
-
Protein histidine phosphatase
- ROS:
-
Reactive oxygen species
- ZDF rat:
-
Zucker diabetic fatty rat
References
Alex LA, Simon MI (1994) Protein histidine kinases and signal transduction in prokaryotes and eukaryotes. Trends Genet 10:133–138
Alves Cde A, Aguiar RA, Alves AC, Santana MA (2007) Diabetes mellitus in patients with cystic fibrosis. J Bras Pneumol 33:213–221
An R, Chu YL, Tian C, Dai XX, Chen JH, Shi Q, Han J, Dong XP (2008) Over-expression of nm23-H1 in HeLa cells provides cells with higher resistance to oxidative stress possibly due to raising intracellular p53 and GPX1. Acta Pharmacol Sin 29:1451–1458
Arnaud-Dabernat S, Bourbon PM, Dierich A, Le Meur M, Daniel JY (2003) Knockout mice as a model systems for studying nm23/NDP kinase gene functions. Application to the nm23-M1 gene. J Bioenerg Biomembranes 35:19–30
Becker KA, Tummler B, Gulbins E, Grassme H (2010a) Accumulation of ceramide in the trachea and intestine of cystic fibrosis mice causes inflammation and cell death. Biochem Biophys Res Commun 403:368–374
Becker KA, Riethmuller J, Luth A, Doring G, Kleuser B, Gulbins E (2010b) Acid sphingolyelinase inhibitors normalize pulmonary ceramide and inflammation in cystic fibrosis. Am J Respir Cell Mol Biol 42:716–724
Becker KA, Riethmuller J, Zhang Y, Gulbins E (2010c) The role of sphingolipids and ceramide in pulmonary inflammation in cystic fibrosis. Open Respir Med J 4:39–47
Besant PG, Attwood PV (2005) Mammalian histidine kinases. Biochim Biophys Acta 1754:281–290
Cuello F, Schulze RA, Heemeyer F, Meyer HE, Lutz S, Jakobs KH, Niroomand F, Wieland T (2003) Activation of heterotrimeric G proteins by a high energy phosphate transfer via nucleoside diphosphate kinase (NDPK) and Gβ subunits. Complex formation of NDPK B with Gβγ dimers and phosphorylation of His-266 in Gβ. J Biol Chem 278:7220–7226
Di L, Srivastava S, Zhdanova O, Sun Y, Li Z, Skolnik EY (2010) Nucleoside diphosphate kinase B knock-out mice have impaired activation of the K+ channel Kca3.1, resulting in defective T cell activation. J Biol Chem 285:38765–38771
Gomes-Alves P, Couto F, Pesquita C, Coelho AV, Penque D (2010) Rescue of F508del-CFTR by RXR motif inactivation triggers proteome modulation associated with unfolded protein response. Biochim Biophys Acta 1804:856–865
Guay C, Madiraju SR, Aumais A, Joly E, Prentki M (2007) A role for ATP citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J Biol Chem 282:35657–35665
Gulbins E (2010) Lipids control mucus production in cystic fibrosis. Nat Med 16:267–268
Higashijima T, Uzu S, Nakajima T, Ross EM (1988) Mastoparan, a peptide toxin from wasp venom mimics receptors by activating GTP-binding regulatory proteins (G-proteins). J Biol Chem 263:6491–6494
Higashijima T, Burnier J, Ross EM (1990) Regulation of Gi and Go by mastoparan, related amphiphilic peptides, and hydrophobic amines. J Biol Chem 265:14176–14181
Hippe HJ, Lutz S, Cuello F, Knorr K, Vogt A, Jakobs KH, Wieland T, Niroomand F (2003) Activation of heterotrimeric G proteins by a high energy phosphate transfer via nucleoside diphosphate kinase (NDPK) and Gβ subunits. Specific activation of Gsα by an NDPK B-Gβγ complex in H10 cells. J Biol Chem 278:7227–7233
Hippe HJ, Wolf NM, Abu-Taha I, Mehringer R, Just S, Lutz S, Niroomand F, Postel EH, Katus HA, Rottbauer W, Wieland T (2009) The interaction of nucleoside diphosphate kinase B with Gβγ dimers controls hetrotrimeric G protein activation. Proc Natl Acad Sci USA 106:16269–16274
Hippe HJ, Abu-Taha I, Wolf NM, Katus HA, Wieland T (2011) Through scaffolding and catalytic actions nucleoside diphosphate kinase B differentially regulates basal and β-adrenoreceptor-stimulated cAMP synthesis. Cell Signal 23:579–585
Jones PM, Persaud SJ (1998) Protein kinases, protein phosphorylation, and the regulation of insulin secretion from pancreatic β-cells. Endocr Rev 19:429–461
Joseph JW, Odegaard ML, Ronnebaum SM, Burgess SC, Muehlbauer J, Sherry AD, Newgard CB (2007) Normal flux through ATP-citrate lyase or fatty acid synthase is not required for glucose-stimulated insulin secretion. J Biol Chem 282:31592–31600
Kamath V, Kyathanahalli CN, Jayaram B, Syed I, Olson K, Ludwig K, Klumpp S, Krieglstein J, Kowluru A (2010) Regulation of glucose- and mitochondrial fuel-induced insulin secretion by a cytosolic protein histidine phosphatase in pancreatic beta-cells. Am J Physiol Endocrinol Metab 299:E276–E286
Klumpp S, Krieglstein J (2002) Phosphorylation and dephosphorylation of histidine residues in proteins. Eur J Biochem 269:1067–1071
Klumpp S, Krieglstein J (2009) Reversible phosphorylation of histidine residues in proteins from vertebrates. Sci Signal 2:pe13
Kowluru A (2002) Identification and characterization of a novel protein histidine kinase in the islet β-cell: evidence for its regulation by mastoparan, an activator of G-proteins and insulin secretion. Biochem Pharmacol 63:2091–2100
Kowluru A (2003a) Defective protein histidine phosphorylation in islets from the Goto-Kakizaki diabetic rat. Am J Physiol Endocrinol Metab 285:E498–E503
Kowluru A (2003b) Regulatory roles for small G-proteins in the pancreatic beta cell: lessons from models of impaired insulin secretion. Am J Physiol Endocrinol Metab 285:E669–E684
Kowluru A (2005) Novel regulatory roles for protein phosphatase-2A in the islet beta cell. Biochem Pharmacol 69:1681–1691
Kowluru A (2008) Emerging roles for protein histidine phosphorylation in cellular signal transduction: lessons from the islet beta-cell. J Cell Mol Med 12:1885–1908
Kowluru A (2011) Friendly, and not so friendly, roles of Rac1 in islet β-cell function: lessons learnt from pharmacological and molecular biological approaches. Biochem Pharmacol. doi:10.1016/j.bcp.2011.01.013
Kowluru A, Metz SA (1994) Characterization of nucleoside diphosphokinase activity in human and rodent pancreatic β cells: evidence for its role in the formation of guanosine triphosphate, a permissive factor for nutrient-induced insulin secretion. Biochemistry 33:12495–12503
Kowluru A, Seavey SE, Rhodes CJ, Metz SA (1996a) A novel regulatory mechanism for trimeric GTP-binding proteins in the membrane and secretory granule fractions of human and rodent beta-cells. Biochem J 313:97–108
Kowluru S, Seavey SE, Li G, Sorenson RL, Weinhaus AJ, Nesher R, Rabaglia ME, Vadakekalam J, Metz SA (1996b) Glucose- and GTP-dependent stimulation of the carboxyl methylation of Cdc42 in rodent and human pancreatic islets and pure beta cells. Evidence for an essential role of GTP-binding proteins in nutrient-induced insulin secretion. J Clin Invest 98:540–555
Kowluru A, Li G, Metz SA (1997) Glucose activates the carboxylmethylation of gamma subunits of trimeric GTP-binding proteins in pancreatic beta cells. Modulation in vivo by calcium, GTP, and pertussis toxin. J Clin Invest 100:1596–1610
Kowluru A, Tannous M, Chen HQ (2002) Localization and characterization of the mitochiondrial isoform of the nucleoside diphosphate kinase in the pancreatic β-cell: evidence for its complexation with mitochondrial succinyl-CoA synthetase. Arch Biochem Biophys 398:160–169
Kowluru A, Veluthakal R, Kaetzel DM (2006) Regulatory roles for nm23/nucleoside diphophate kinase-like enzymes in insulin secretion from the pancreatic islet beta cell. J Bioenerg Biomembr 38:227–232
Laguna TA, Nathan BM, Moran A (2010) Managing diabetes in cystic fibrosis. Diab Obes Metab 12:858–864
Le E, Jeong J, Kim SE, Song EJ, Kang SW, Lee K-J (2009) Multiple functions of nm23-H1 are regulated by oxido-reduction system. PlosOne 4:1–14
MacDonald MJ (1990) Elusive proximal signals of beta-cells for insulin secretion. Diabetes 39:1461–1466
MacDonald MJ, Smith AD III, Hasan NM, Sabat G, Fahein LA (2007) Feasibility of pathways for transfer of acyl groups from mitochondria to the cytosol to form short chain acyl-CoAs in the pancreatic beta cell. J Biol Chem 282:30596–30606
Matthews HR (1995) Protein kinases and phosphatases that act on histidine, lysine, or arginine residues in eukaryotic proteins: a possible regulator of the mitogen activated protein kinase cascade. Pharmacol Ther 67:323–350
Metz SA (1991) The pancreatic islet as a Rubik’s cube. Is phospholipid hydrolysis a piece of the puzzle. Diabetes 40:1565–1573
Metz SA, Rabaglia ME, Pintar (1992) Selective inhibitors of GTP synthesis impede exocytotic insulin release from intact rat islets. J Biol Chem 267:12517–12527
Metz SA, Meredith M, Rabaglia ME, Kowluru A (1993) Small elevations of glucose concentration redirect and amplify the synthesis of guanosine 5′-triphosphate in rat islets. J Clin Invest 92:872–882
Metz SA, Meredith ME, Vadakekalam J, Rabaglia ME, Kowluru A (1999) A defect late in stimulus-secretion coupling impairs insulin secretion in Goto Kakizaki diabetic rats. Diabetes 48:1754–1762
Nesher R, Anteby E, Yedozvizky M, Warwar N, Cerasi E (2002) Beta-cell protein kinases and the dynamics of the insulin response to glucose. Diabetes 51(suppl 1):S63–S73
Newgard CB, McGarry JD (1995) Metabolic coupling factors in pancreatic β-cell signal transduction. Annu Rev Biochem 64:689–719
Pia EK, Pettersson G, Bo EK, Gong F, Li JP, Zetterqvist O (2002) Identification and characterization of mammalian 14-kDa phosphohistidine phosphatase. Eur J Biochem 269:5016–5023
Prentki M, Matschinsky FM (1987) Calcium, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev 67:1223–1226
Steeg PS, Palmieri D, Ouatas T, Salerno M (2003) Histidine kinases and histidine phosphorylated proteins in mammalian cell biology, signal transduction and cancer. Can Lett 190:1–12
Subasinghe I, Syed I, Kowluru A (2011) Phagocyte-like NADPH oxidase promotes cytokine mitochondrial dysfunction in pancreatic beta-cells: evidence for regulation by Rac1. Am J Physiol Regul Integr Comp Physiol 300:R21–20
Syed I, Jayaram B, Subasinghe W, Kowluru A (2010) Tiam1/Rac1 signaling pathway mediates palmitate-induced, ceramide-sensitive generation of superoxides and lipid hydroperoxides and the loss in mitochondrial membrane potential in pancreatic beta-cells. Biochem Pharmacol 80:874–883
Tofe S, Moreno JC, Maiz L, Alonso M, Escobar H, Barrio R (2005) Insulin-secretion abnormalities and clinical deterioration related to impaired glucose tolerance in cystic fibrosis. Eur J Endocrinol 152:241–247
Veluthakal R, Palanivel R, Zhao Y, McDonald P, Gruber S, Kowluru A (2005) Ceramide induces mitochondrial abnormalities in insulin-secreting INS-1 cells: potential mechanisms underlying ceramide-mediated metabolic dysfunction of the beta-cell. Apoptosis 10:841–850
Veluthakal R, Suresh MV, Kowluru A (2009) Down-regulation of expression and function of nucleodide diphosphate kinase in insulin-secreting beta-cells under in vitro conditions of glucolipotoxicity. Mol Cell Biochem 329:121–129
Wieland T, Nunberg B, Ulibarri I, Kaldenberg-Stasch S, Schultz G, Jakobs KH (1993) Guanine nucleotide-specific phosphate transfer by guanine-binding regulatory protein beta-subunits. Characterization of phosphorylated amino acids. J Biol Chem 268:18111–18118
Wieland T, Hippe HJ, Ludwig K, Zhou XB, Korth M, Klumpp S (2010) Reversible histidine phosphorylation in mammalian cells: a teeter-totter formed by nucleoside diphosphate kinase and protein histidine phosphatase 1. Meth Enzymol 471:379–402
Acknowledgements
This research was supported by a Merit Review Award from the Department of Veterans Affairs and the National Institutes of Health [DK 74921]. AK is also the recipient of the Senior Research Career Scientist Award from the Department of Veterans Affairs.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is dedicated to the late Dr. Susanne Klumpp for her pioneering contributions to the field of protein histidine [de]phosphorylation in cellular signal transduction.
Rights and permissions
About this article
Cite this article
Kowluru, A., Klumpp, S. & Krieglstein, J. Protein histidine [de]phosphorylation in insulin secretion: abnormalities in models of impaired insulin secretion. Naunyn-Schmiedeberg's Arch Pharmacol 384, 383–390 (2011). https://doi.org/10.1007/s00210-011-0616-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-011-0616-z