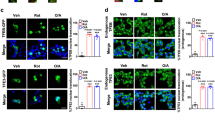
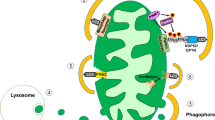
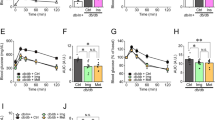
C2-ceramide, a cell permeable analogue of ceramide [CER] markedly reduced mitochondrial membrane potential [MMP] in insulin-secreting INS cells, which was followed by a significant accumulation of cytochrome c [Cyt c] into the cytosolic compartment. In a manner akin to CER, exposure of these cells to interleukin-1β [IL-1β] also resulted in reduction in MMP and cytosolic accumulation of Cyt c. Further, long-term exposure of these cells to either CER [but not its inactive analogue] or IL-1β caused a marked reduction in their metabolic viability. However, unlike IL-1β, which increased nitric oxide [NO] release, CER-treatment of INS cells had no effects of CER on NO release were demonstrable. Together, these findings suggest that CER-induced mitochondrial effects may not be mediated via iNOS gene expression and NO production. CER also activated an okadaic acid -sensitive protein phosphatase [CAPP] in the purified mitochondrial fraction, suggesting that CAPP might represent one of the target proteins for CER in the β cell mitochondria. Together, our findings suggest direct detrimental effects of CER on mitochondrial function in β cells leading to their dysfunction and demise via apoptosis. Moreover, our findings provide evidence for a potential difference in the mechanisms underlying CER- and IL-1β-induced mitochondrial defects and apoptotic demise of the effete β cell.
Similar content being viewed by others
References
Cuvillier O. Sphingosine in apoptosis signaling. Biochim Biophys Acta 2002; 1585: 153–162.
Choi MS, Anderson MA, Zhang Z, Zimonjic DB, Popescu N, Mukherjee AB. Neutral ceramidase gene: role in regulating ceramide-induced apoptosis. Gene 2003; 315: 113–122.
Dbaibo GS, Hannun YA. Signal transduction and the regulation of apoptosis: roles of ceramide. Apoptosis 1998; 3: 317–334.
Gentil B, Grimot F, Riva C. Commitment to apoptosis by ceramides depends on mitochondrial respiratory function, cytochrome c release and caspase-3 activation in Hep-G2 cells. Mol Cell Biochem 2003; 254: 203–210.
Sillence DJ. Apoptosis and signalling in acid sphingomyelinase deficient cells. BMC cell Biol 2001; 2: 24.
Maceyka M, Payne SG, Milstein S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta 2002; 1585: 193–201.
Kowluru A, Metz SA. Ceramide-activated protein phosphatase-2A activity in insulin-secreting cells. FEBS Lett 1997; 418: 179–182.
Ruvolo PP. Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol Res 2003; 47: 383–392.
Ruvolo PP, Clark W, Mumby M, Gao F, May WS. A functional role for the B56α alpha-subunit of protein phosphatase 2A in ceramide-mediated regulation of Bcl2 phosphorylation status and function. J Biol Chem 2002; 277: 22847–22852.
Ruvolo PP, Deng X, Ito T, Carr BK, May WS. Ceramide induces Bcl2 dephosphorylation via a mechanism involving mitochondrial PP2A. J Biol Chem 1999; 274: 20296–20300.
Ito T, Deng X, Carr B, May WS. Bcl-2 phosphorylation is required for anti-apoptosis function. J Biol Chem 1997; 272: 11671–11673.
Ruvolo PP, Deng X, May WS. Phosphorylation of Bcl-2 and regulation of apoptosis. Leukemia 2001; 15: 515–522.
Shimizu H, Okajima F, Kimura T et al. Sphingosine 1-phosphate stimulates insulin secretion in HIT-T 15 cells and mouse islets. Endocr J 2000; 47: 261–269.
Birbes H, Luberto C, Hsu YT, Bawab SE, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNF alpha induced Bax translocation to mitochondria. Biochem J 2004; [Epub ahead of print].
Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem 2003; 278: 30015–30021.
Lupi R, Dotta F, Marselli L, et al. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that β cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes 2002; 51: 1437–1442.
Major CD, Gao ZY, Wolf BA. Activation of the sphingomyelinase/ceramide signal transduction pathway in insulin-secreting beta-cells: role in cytokine-induced beta- β cell death. Diabetes 1999; 48: 1372–1380.
Shimabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem 1998; 273: 32487–32490.
Sjoholm A. Ceramide inhibits pancreatic beta-cell insulin production and mitogenesis and mimics the actions of interleukin-1 beta. FEBS Lett 1995; 367: 283–286.
Sjoholm A. Aspects of the involvement of interleukin-1 and nitric oxide in the pathogenesis of insulin-dependent diabetes mellitus. Cell Death Differ 1998; 5: 461–468.
Welsh N. Interleukin fl-induced ceramide and diacylglycerol generation may lead to activation of the c-Jun NH2-terminal kinase and transcription factor ATF2 in the insulin-producing cell line RINm5F. J Biol Chem 1996; 271: 8307–8312.
Ishizuka N, Yagui K, Tokuyama Y, et al. Tumor necrosis factor alpha signaling pathway and apoptosis in pancreatic beta cells. Metabolism 1999; 48: 1485–1492.
Kwon G, Bohrer A, Han X, et al. Characterization of the sphingomyelin content of isolated pancreatic islets. Evaluation of the role of sphingomyelin hydrolysis in the action of interleukin-1 to induce islet overproduction of nitric oxide. Biochim Biophys Acta 1996; 1300: 63–72.
Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N Donath MY. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes 2001; 50: 69–76.
Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta 2002; 1585: 202–212.
Kwon G, Pappan KL, Marshall CA, Schaffer JE, McDaniel ML. Cyclic AMP dose-dependently prevents palmitate-induced apoptosis by both PKA-and cAMP-GEF-dependent pathways in beta cells. J Biol Chem 2004; 279: 8938–8945.
Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998; 95: 2498–2502.
Di Paola M, Zaccagnino P, Montedoro G, Cocco T, Lorusso M. Ceramide induces release of pro-apoptotic proteins from mitochondria by either a Ca2+ -dependent or a Ca2+ -independent mechanism. J Bioenerg Biomembr 2004; 36: 165–170.
Birbes H, Bawab SE, Obeid LM, Hannun YA. Mitochondria and ceramide: intertwined roles in regulation of apoptosis. Adv Enzyme Regul. 2002; 42: 113–129.
Kowluru A, Tannous M, Chen HQ. Localization and characterization of the mitochondrial isoform of the nucleoside diphosphate kinase in the pancreatic beta β cell: evidence for its complexation with mitochondrial succinyl-CoA synthetase. Arch Biochem Biophys 2002; 398: 160–169.
Tannous M, Amin R, Popoff MR, Fiorentini C, Kowluru A. Positive modulation by Ras of interleukin-1beta-mediated nitric oxide generation in insulin-secreting clonal beta (HIT-T15) cells. Biochem Pharmacol 2001; 62: 1459–1468.
Kowluru A, Seavey S Li G et al. Glucose- and GTP-dependent stimulation of the carboxylmethylation of CDC42 in rodent and human pancreatic islets and pure beta β cells. Evidence for an essential role of GTP-binding proteins in nutrient-induced insulin secretion. J Clin Invest 1996; 98: 540–555.
Kowluru A, Li G, Metz SA. Glucose activates the carboxylmethylation of gamma subunits of trimeric GTP-binding proteins in pancreatic beta cells. Modulation in vivo by calcium, GTP, and pertussis toxin. J Clin Invest 1997; 100: 596–610.
Favre B, Zolnierowicz S, Turowski P, Hemmings BA. The catalytic subunit of protein phosphatase 2A in carboxylmethylated in vivo. J Biol Chem 1994; 269: 16311–16317.
Xie H, Clarke S. Protein phosphatase 2A is reversibly modified by methyl esterification at its C-terminal leucine residue in bovine brain. J Biol Chem 1994; 269: 1981–1984.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254.
van Blitterswijk WJ, van der Luit AH, Veldman RJ, Verheij M, Borst J. Ceramidel: second messenger or modulator of membrane structure and dynamics?. Biochem J. 2003; 369: 199–211.
Eizirik DL, Cagliero E, Bjorklund A, Welsh N. Interleukin-1 beta induces the expression of an isoform of nitric oxide synthase in insulin-producing cells, which is similar to that observed in activated macrophages. FEBS Lett 1992; 308: 249–252.
Kwon G, Corbett JA, Rodi CP, Sullivan P, McDaniel ML. Interleukin-1 beta-induced nitric oxide synthase expression by rat pancreatic beta-cells: evidence for the involvement of nuclear factor kappa B in the signaling mechanism. Endocrinology. 1995; 136: 4790–4795.
Reimers JI, Andersen HU, Mauricio D, et al. Strain-dependent differences in sensitivity of rat beta-cells to interleukin 1 beta in vitro and in vivo: association with islet nitric oxide synthesis. Diabetes 1996; 45: 771–778.
Chen HQ, Tannous M, Veluthakal R, Amin R, Kowluru A. Novel roles for palmitoylation of Ras in IL-1β beta-induced nitric oxide release and caspase 3 activation in insulin-secreting beta cells. Biochem Pharmacol 2003; 66: 1681–1694.
Zolnierowicz S: Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem Pharmacol 2000; 60: 1225–1235.
Deng X, Gao F, Flagg T May WS, Jr. Mono- and multisite phosphorylation enhances Bcl2’s antiapoptotic function and inhibition of cell cycle entry functions. Proc Natl Acad Sci USA 2004; 101: 153–158.
Jiffar T, Kurinna S, Suck G et al. PKC alpha mediates chemoresistance in acute lymphoblastic leukemia through effects on Bcl2 phosphorylation. Leukemia 2004; 18: 505–512.
Tran VV, Chen G, Newgard CB, Hohmeier HE. Discrete and complementary mechanisms of protection of beta cells against cytokine-induced and oxidative damage achieved by bcl-2 overexpression and a cytokine selection strategy. Diabetes 2003; 52:1423–1432.
Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem 1993; 268: 15523–15530.
Galadari S, Kishikawa K, Kamibayashi C, Mumby MC, Hannun YA. Purification and characterization of ceramide-activated protein phosphatases. Biochemistry 1998; 37: 11232–11238.
Bourbon NA, Yun J, Kester M. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J Biol Chem 2000; 275: 35617–35623.
Zhang Y, Yao B, Delikat S et al. Kinase suppressor of Ras is ceramide-activated protein kinase. Cell 1997; 89: 63–72.
Kowluru A, Seavey SE, Rabaglia ME, Nesher R, Metz SA. Carboxylmethylation of the catalytic subunit of protein phosphatase 2A in insulin-secreting cells: Evidence for functional consequences on enzyme activity and insulin secretion. Endocrinology 1996; 137: 2315–2323.
El-Assaad W, Buteau J, Peyot ML, et al. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology 2003; 144: 4154–163.
Garcia A, Cayla X, Guergnon J, et al. Serine/threonine protein phosphatases PP1 and PP2A are key players in apoptosis. Biochimie 2003; 85: 721–726.
Klumpp S, Selke D, Krieglstein J. Protein phosphatase type 2C dephosphorylates BAD. Neurochem Int 2003; 42: 555–560.
Chalfant CE, Szulc Z, Roddy P, Bielawska A, Hannun YA. The structural requirements for ceramide activation of serine-threonine protein phosphatases. Lipid Res 2004; 45: 496–506.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Veluthakal, R., Palanivel, R., Zhao, Y. et al. Ceramide induces mitochondrial abnormalities in insulin-secreting INS-1 cells: Potential mechanisms underlying ceramide-mediated metabolic dysfunction of the β cell. Apoptosis 10, 841–850 (2005). https://doi.org/10.1007/s10495-005-0431-4
Issue Date:
DOI: https://doi.org/10.1007/s10495-005-0431-4