Abstract
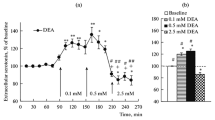
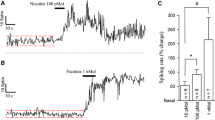
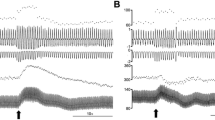
To elucidate conflicting findings about the role of l-arginine/nitric oxide (NO) pathway in the locus coeruleus (LC), we investigated the effects of different drugs affecting NO concentrations by single-unit extracellular recordings from LC neurons in vivo and in vitro. In anesthetized rats, central (3.8–15.3 nmol i.c.v.) and local (16.5–66 pmol into the LC) administrations of the NO donor sodium nitroprusside, but not those of the inactive analogue potassium ferricyanide (16.5–66 pmol into the LC), increased by 65–84% the firing rate of LC neurons. In brain slices, low concentrations (50–200 μM) of diethylamine/NO complex, a short-lived NO releaser, also increased the neuron firing rate, although higher drug concentrations (400–800 μM) caused slowly reversible reductions of the firing activity. On the other hand, the NO synthase inhibitors Nω-nitro-l-arginine methyl ester (l-NAME) (148–371 nmol i.c.v.) and Nω-nitro-l-arginine (l-NA) (46 nmol i.c.v.) gradually decreased the firing rate of LC neurons, whereas the NO synthase substrate l-arginine (0.71–1.42 μmol i.c.v. and 0.6–4.8 nmol into the LC) increased the neuron activity. The latter effect was not mimicked by the vehicle or the less active isomer d-arginine (0.6–4.8 nmol into the LC). Unexpectedly, pretreatment with high concentrations of l-NAME (371 nmol and 18.5 μmol i.c.v.) or l-NA (45.6 nmol i.c.v. and 0.24 nmol into the LC) failed to block the effect of l-arginine. The glutamate receptor antagonist kynurenic acid (1 μmol i.c.v.) strongly reduced the effect of l-arginine but not that of sodium nitroprusside. These data confirm in vivo a direct excitatory effect of NO on LC neurons and suggest a tonic regulation of noradrenergic neurons by NO in vivo. l-arginine also excites LC neurons, but this effect may be caused by a nitric-oxide-unrelated glutamate-receptor-mediated mechanism.






Similar content being viewed by others
References
Alreja M, Aghajanian GK (1995) Intracellular application of macromolecules through patch pipettes in brain slices. In: Schurr A, Rigor BM (eds) Brain slices in basic and clinical research. CRC Press, London, pp 117–130
Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450
Colasanti M, Suzuki H (2000) The dual personality of NO. Trends Pharmacol Sci 21:249–252
Cox BA, Johnson SW (1998) Nitric oxide facilitates N-methyl-D-aspartate-induced burst firing in dopamine neurons from rat midbrain slices. Neurosci Lett 255:131–134
Cuellar B, Fernandez AP, Lizasoain I, Moro MA, Lorenzo P, Bentura ML, Rodrigo J, Leza JC (2000) Up-regulation of neuronal NO synthase immunoreactivity in opiate dependence and withdrawal. Psychopharmacology 148:66–73
Curtis AL, Drolet G, Valentino RJ (1993) Hemodynamic stress activates locus coeruleus neurons of unanesthetized rats. Brain Res Bull 31:737–744
Dawson TM, Dawson VL (1996) Nitric oxide synthase: role as a transmitter/mediator in the brain and endocrine system. Ann Rev Med 47:219–227
De Vente J, Steinbusch HWM (2000) Nitric oxide-cGMP signalling in the rat brain. In: Steinbusch HWM, De Vente J (eds) Handbook of chemical neuroanatomy: functional neuroanatomy of the nitric oxide system. Elsevier Science B.V, Amsterdam, pp 355–415
Desvignes C, Robert F, Vachette C, Cespuglio R, Renaud B, Lambas-Señas L (1997) Monitoring nitric oxide (NO) in rat locus coeruleus: differential effects of NO synthase inhibitors. Neuroreport 8:1321–1325
El-Husseini AE, Bladen C, Vincent SR (1995) Molecular characterization of a type II cyclic GMP-dependent protein kinase expressed in the rat brain. J Neurochem 64:2814–2817
Ennis M, Aston-Jones G, Shierkhattar R (1992) Activation of locus coeruleus neurons by nucleus paragigantocellularis or noxious sensory stimulation is mediated by intracoerulear excitatory amino acid neurotransmission. Brain Res 598:185–195
Fabris G, Steiner AA, Anselmo-Franci JA, Branco LG (2000) Role of nitric oxide in rat locus coeruleus in hypoxia-induced hyperventilation and hypothermia. Neuroreport 11:2991–2995
Feelisch M (1998) The use of nitric oxide donors in pharmacological studies. Naunyn-Schmiedeberg’s Arch Pharmacol 358:113–122
Furuyama T, Inagaki S, Takagi H (1993) Localizations of alpha-1 and beta-1 subunits of soluble guanylate cyclase in the rat brain. Mol Brain Res 20:335–344
Garthwaite J (2000) The physiological roles of nitric oxide in the central nervous system. In: Mayer B (ed) Handbook of experimental pharmacology: nitric oxide. Springer, Berlin, pp 259–275
Hall S, Milne B, Jhamandas K (1996) Nitric oxide synthase inhibitors attenuate acute and chronic morphine withdrawal response in the rat locus coeruleus: an in vivo voltammetric study. Brain Res 739:182–191
Hall S, Milne B, Jhamandas K (1998) Excitatory action of N-methyl-D-aspartate on the rat locus coeruleus is mediated by nitric oxide: an in vivo voltammetric study. Brain Res 796:176–186
Javelle N, Berod A, Renaud B, Lambas-Señas L (2002) NO synthase inhibitors attenuate locus coeruleus catecholamine metabolism and behavior induced by morphine withdrawal. Neuroreport 13:725–728
Kawabata A, Umeda N, Takagi H (1993) L-arginine exerts a dual role in nociceptive processing in the brain: involvement of the kyotorphin-Met-enkephalin pathway and NO-cyclic GMP pathway. Br J Pharmacol 109:73–79
Kiss JP, Vizi ES (2001) Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci 24:211–215
Krukoff TL (1999) Central actions of nitric oxide in regulation of autonomic functions. Brain Rev Rev 30:52–65
Lipton P, Aitken PG, Dudek FE, Eskessen K, Espanol MT, Ferchmin PA, Kelly JB, Kreisman NR, Landfield PW, Larkman PM (1995) Making the best of brain slices: comparing preparative methods. J Neurosci Meth 59:151–156
Lohse MJ, Forstermann U, Schmidt HH (1998) Pharmacology of NO:cGMP signal transduction. Naunyn-Schmiedeberg’s Archiv Pharmacol 358:111–112
Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, Bove AA, Isaac L, Hrabie JA, Keefer LK (1991) Complexes of NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J Med Chem 34:3242–3247
Milne B, Hall SR, Sullivan ME, Loomis C (2001) The release of spinal prostaglandin E2 and the effect of nitric oxide synthetase inhibition during strychnine-induced allodynia. Anesth Analg 93:728–733
Minc-Golomb D, Yadid G, Tsarfaty I, Resau JH, Schwartz JP (1996) In vivo expression of inducible nitric oxide synthase in cerebellar neurons. J Neurochem 66:1504–1509
Moore PK, Handy RL (1997) Selective inhibitors of neuronal nitric oxide synthase-is no NOS really good NOS for the nervous system? Trends Pharmacol Sci 18:204–211
Moore PK, Oluyomi AO, Babbedge RC, Wallace P, Hart SL (1991) L-NG-nitro arginine methyl ester exhibits antinociceptive activity in the mouse. Br J Pharmacol 102:198–202
Nurminen ML, Vapaatalo H (1996) Effect of intracerebroventricular and intravenous administration of nitric oxide donors on blood pressure and heart rate in anaesthetized rats. Br J Pharmacol 119:1422–1426
Pineda J, Kogan JH, Aghajanian GK (1996) Nitric oxide and carbon monoxide activate locus coeruleus neurons through a cGMP-dependent protein kinase: involvement of a nonselective cationic channel. J Neurosci 16:1389–1399
Pineda J, Torrecilla M, Martín-Ruiz R, Ugedo L (1998) Attenuation of withdrawal-induced hyperactivity of locus coeruleus neurones by inhibitors of nitric oxide synthase in morphine-dependent rats. Neuropharmacology 37:759–767
Prast H, Philippu A (2001) Nitric oxide as modulator of neuronal function. Prog Neurobiol 64:51–68
Ruiz-Durantez E, Ruiz-Ortega JA, Pineda J, Ugedo L (2002) Effect of agmatine on locus coeruleus neuron activity: possible involvement of nitric oxide. Br J Pharmacol 135:1152–1158
Salter M, Duffy C, Garthwaite J, Strijbos PJ (1995) Substantial regional and hemispheric differences in brain nitric oxide synthase (NOS) inhibition following intracerebroventricular administration of N omega-nitro-L-arginine (L-NA) and its methyl ester (L-NAME). Neuropharmacology 34:639–649
Silva MT, Rose S, Hindmarsh JG, Jenner P, Marsden CD (1998) L-arginine produces NO-independent increases in dopamine efflux in rat striatum. Neuroreport 9:149–152
Smith PA, Sakura H, Coles B, Gummerson N, Proks P, Ashcroft FM (1997) Electrogenic arginine transport mediates stimulus-secretion coupling in mouse pancreatic beta-cells. J Physiol 499:625–635
Snyder SH, Jaffrey SR, Zakhary R (1998) Nitric oxide and carbon monoxide: parallel roles as neural messengers. Brain Res Rev 26:167–175
Southam E, Garthwaite J (1991) Comparative effects of some nitric oxide donors on cyclic GMP levels in rat cerebellar slices. Neurosci Lett 130:107–111
Tassorelli C, Joseph SA, Buzzi MG, Nappi G (1999) The effects on the central nervous system of nitroglycerin–putative mechanisms and mediators. Prog Neurobiol 57:607–624
Torrecilla M, Pineda J, Ugedo L (2001) NO synthase inhibitors reduce opioid desensitization in rat locus coeruleus neurons in vitro. Neuroreport 12:1601–1604
Uzbay IT, Oglesby MW (2001) Nitric oxide and substance dependence. Neurosci Biobehav Rev 25:43–52
Vincent SR, Kimura H (1992) Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience 46:755–784
Vulliemoz Y, Whittington RA, Virag L (1999) The nitric oxide-cGMP system of the locus coeruleus and the hypnotic action of alpha-2 adrenergic agonists. Brain Res 849:169–174
Wiesinger H (2001) Arginine metabolism and the synthesis of nitric oxide in the nervous system. Prog Neurobiol 64:365–391
Xu ZQ, Pieribone VA, Zhang X, Grillner S, Hokfelt T (1994) A functional role for nitric oxide in locus coeruleus: immunohistochemical and electrophysiological studies. Exp Brain Res 98:75–83
Xu ZQ, De-Vente J, Steinbusch H, Grillner S, Hokfelt T (1998) The NO-cGMP pathway in the rat locus coeruleus: electrophysiological, immunohistochemical and in situ hybridization studies. Eur J Neurosci 10:3508–3516
Yao ST, Finkelstein DI, Lawrence AJ (1999) Nitrergic stimulation of the locus coeruleus modulates blood pressure and heart rate in the anaesthetized rat. Neuroscience 91:621–629
Ye S, Nosrati S, Campese VM (1997) Nitric oxide (NO) modulates the neurogenic control of blood pressure in rats with chronic renal failure (CRF). J Clin Invest 99:540–548
Acknowledgments
This work was supported by Ministerio de Ciencia y Tecnología (MCT) (SAF 99/0046 and SAF 2001/0552), Ministerio de Salud y Consumo (MSC) (PI 05/0513, RTA G03/005 and PND-MSC 2005), the Basque Government (PE 04UN12), University of the Basque Country (026.327–13590/2001 and 0026.327-E-15924/2004). M. Torrecilla was supported by a fellowship from the UPV-EHU.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torrecilla, M., Ruiz-Ortega, J.A., Ugedo, L. et al. Excitatory regulation of noradrenergic neurons by l-arginine/nitric oxide pathway in the rat locus coeruleus in vivo. Naunyn-Schmied Arch Pharmacol 375, 337–347 (2007). https://doi.org/10.1007/s00210-007-0163-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-007-0163-9