Abstract
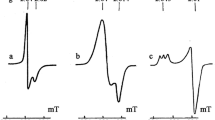
Spin trapping experiments have been carried out on solutions generated by the dissolution of nickel oxides, all of which contained NiO (bunsenite) as the predominant crystalline phase. Samples containing nickel (III), of low crystallinity, with small particle size and high surface areas can in the presence of H2O2, cause the generation of the hydroxyl radical (as detected by spin trapping with 5,5-dimethyl-1-pyrroline N-oxide or DMPO) and/or another highly reactive species capable of cleaving the C-S bond in dimethyl sulphoxide. In contrast samples which predominantly contain nickel (II), of high crystallinity, with a large particle size and low surface area cause the generation of alkyl and alkoxyl radicals, or another highly reactive species capable of causing the decomposition of lipid peroxides, but did not generate significant quantities of the hydroxy radical type. These findings help to explain the varied ability of nickel containing solids to cause DNA lesions in vivo and in vitro.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 28 November 1995/Accepted: 22 March 1996
Rights and permissions
About this article
Cite this article
O’Brien, P., Salacinski, H. Evidence for a relationship between the generation of reactive intermediates and the physicochemical characteristics of nickel oxides. Arch Toxicol 70, 787–800 (1996). https://doi.org/10.1007/s002040050341
Issue Date:
DOI: https://doi.org/10.1007/s002040050341