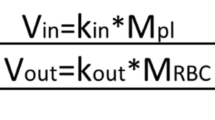Abstract
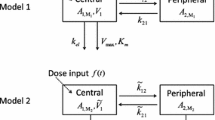
The uptake of methyl mercury (MeHg) by isolated rat erythrocytes was studied at 37°C using MeHg-cysteine (MeHgCySH), MeHg-glutathione (MeHgGSH), MeHg-mercaptalbumin (MeHgMASH) and the mixture of MeHgCySH with MeHgGSH, MeHgCySH with MeHgMASH, MeHgGSH with MeHgMASH at different MeHg concentrations. The measured MeHg concentrations were analyzed according to the Akaike’s information criterion in order to determine the suitable compartment model. After determining a two-compartment model, a model-independent two-compartment model was developed from the kinetics of uptake of MeHg at a concentration of 1 mmol MeHg/l packed erythrocytes using MeHgCySH CySH, MeHgGSH and MeHgMASH, respectively. The developed two-compartment model was validated by predicting the kinetics of uptake of MeHg by rat erythrocytes at different MeHg concentrations and different mixtures of MeHg-complexes. Then, the predicted values were compared with the measured values. The results suggested: 1) MeHg uptake appeared suitable to be described by a two-compartment model, while using MeHgGSH, MeHgMASH, MeHgCySH at lower concentrations and the mixtures of MeHg-complexes; 2) MeHgCySH uptake was slowest among three kinds of MeHg-complexes, although a postulated cysteine-facilitated MeHgCySH transport system might exist in erythrocyte membrane; 3) the mixture of MeHg-complexes might facilitate MeHgCySH uptake; 4) there might be a second MeHg intracellular compartment in rat erythrocytes.
Similar content being viewed by others
References
Anonymous (1987) Report with confidence. Lancet i: 488
Aschner M, Clarkson TW (1988) Uptake of methylmercury in the rat brain: effect of amino acids. Brain Res 462: 31–39
Aschner M, Clarkson TW (1989) Methyl mercury uptake across bovine brain capillary endothelial cells in vitro: the role of amino acids. Pharmacol Toxicol 65: 17–20
Bach RD, Weibel AT (1976) Nuclear magnetic resonance studies on anion-exchange reactions of alkylmercury mercaptides. J Am Chem Soc 98: 6241–6249
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61: 882–888
Bulpitt CJ (1987) Confidence intervals. Lancet i: 494–497
Campbell MJ, Gardner MJ (1988) Calculating confidence intervals for some non-parametric analyses. BMJ 296: 1454–1456
Chen RW, Lacy VL, Whanger PD (1975) Effect of selenium on methylmercury binding to subcellular and soluble proteins in rat tissues. Res Commun Chem Pathol Pharmacol 12: 297–308
Doi R, Kobayashi T (1982) Organ distribution and biological half-time of methylmercury in four strains of mice. J Exp Med 52: 307–314
Doi R, Tagawa M (1983) A study on the biochemical and biological behavior of methylmercury. Toxicol Appl Pharmacol 69: 407–416
Ellory JC, Preston RL, Osotimehin B, Young JD (1983) Transport of amino acids for glutathione biosynthesis in human and dog red cells. Biomed Biochim Acta 42: S48-S52
Gardner MJ, Altman DG (1986) Confidence intervals rather than P values: estimation rather than hypothesis testing. BMJ 292: 746–750
Garcia JD, Yang MG, Wang JHC, Belo PS (1974) Carbon-mercury bond cleavage in blood of rats fed methyl mercuric chloride. Proc Soc Exp Biol Med 146: 66–70
Gaspari F, Ruggenenti P, Torre L, Bertocchi C, Remuzzi, G, Perico N (1993) Failure to predict cyclosporine area under the curve using a limited sampling strategy. Kidney Int 44: 436–439
Greener Y, Kochen JA (1983) In vitro studies on methyl mercury distribution in human blood. Teratology 28: 375–387
Healy MJR (1979) Outliers in clinical chemistry quality-control schemes. Clin Chem 25: 675–677
Heijin M, Oude Elferink RPJ, Jansen PLM (1992) ATP-dependent multispecific organic anion transport system in rat erythrocyte membrane vesicles. Am J Physiol 262: C104-C110
Herbst MD, Goldstein JH (1989) A review of water diffusion measurement by NMR in human red blood cells. Am J Physiol 256: C1097-C1104
Hsu JM, Rubenstein B, Paleker AG (1982) Role of magnesium in glutathione metabolism of rat erythrocytes. J Nutr 112: 488–496
Hughes WL (1957) A physical rationale for the biological activity of mercury and its compounds. Ann NY Acad Sci 65: 454–460
Jacobs MB, Yamaguchi S, Goldwater LD, Gilbert H, (1960) Determination of mercury in blood. Am Ind Hyg Assoc J 21: 475–480
Jocelyn PC (1967) An assay for glutathione in acid solution. Anal Biochem 18: 493–498
Johnston A, Sketris I, Marsden JT, Galustian CG, Fashola T, Taube D, Pepper J, Holt DW (1990) A limited sampling strategy for the measurement of cyclosporine AUC. Transplant Proc 22: 1345–1346
Kerper LE, Ballatori N, Clarkson TW, (1992) Methylmercury transport across the blood-brain barrier by an amino acid carrier. Am J Physiol 262: R761-R765
Kondo T, Dale GL, Beutler E (1980) Glutathione transport by inside-out vesicles from human erythrocytes. Proc Natl Acad Sci USA 77: 6359–6362
Kondon T, Dale GL, Beutler E (1981) Studies on glutathione transport utilizing inside-out vesicles prepared from human erythrocytes. Biophys Acta 645: 132–136
Kondo T, Murao M, Taniguchi N (1982) Glutathione S-conjugate transport using inside-out vesicles from human erythrocytes. Eur J Biochem 125: 551–554
LaBelle EF, Singh SV, Srivastava SK, Awassthi YC (1986a) Dinitrophenyl glutathione efflux from human erythrocytes in primary active ATP-dependent transport. Biochem J 238: 443–449
LaBelle EF, Singh SV, Srivastava SK, Awassthi YC (1986b) Evidence for different transport systems for oxidized glutathione and S-dinitrophenyl glutathione in human erythrocytes. Biochem Biophys Res Commun 139: 538–544
Langman MJS (1986) Towards estimation of confidence intervals. BMJ 292: 716
McIntyre TM, Curthoys NP (1980) The interorgan metabolism of glutathione. Int J Biochem 12: 545–551
McNeil TL, Beck LV (1968) Fluorometric estimation of GSH-OPT. Anal Biochem 22: 431–441
Naganuma A, Imura N (1979) Methylmercury binds to a low molecular weight substance in rabbit and human erythrocytes. Toxicol Appl Pharmacol 47:613–616
Naganuma A, Kogawa Y, Imura N (1980) Behavior of methylmercury in mammalian erythrocytes. Toxicol Appl Pharmacol 54: 405–410
Rabenstein DL, Anvarthusein AI (1982) A proton nuclear magnetic resonance study of the interaction of mercury with intact human erythrocytes. Biochim Biophys Acta 721: 374–384
Rabenstein DL, Fairhurst MT (1975) Nuclear magnetic resonance studies of the solution chemistry of metal complexes. XI. The binding of methylmercury by sulfhydryl-containing amino acids and by glutathione. J Am Chem Soc 97: 2056–2092
Rabenstein DL, Isab AA, Reid RS (1982) A proton nuclear magnetic resonance study of the binding of methylmercury in human erythrocytes. Biochim Biophys Acta 696: 53–64
Saetre R, Rabenstein DL (1978) Determination of cysteine in plasma and urine and homocysteine in plasma by high-pressure liquid chromatography. Anal Biochem 90: 684–692
Simpson RB (1961) Association constants of methylmercury with sulfhydryl and other bases. J Am Chem Soc 83: 4711–4717
Solomon AK, Gill TJ, Gold GL (1956) The kinetics of cardiac glycoside inhibition of potassium transport in human erythrocytes. J Gen Physiol 40: 327–350
Srivastava ST, Beutler E (1968) Accurate measurement of oxidized glutathione content of human, rabbit and rat red blood cells and tissues. Anal Biochem 25: 70–76
Synder RM, Mirabelli CK, Crooke ST (1986) Cellular association, intracellular distribution and efflux of auranofin via sequential ligand exchange reactions. Biochem Pharmacol 38: 923–932
Takeda Y, Kunugi T, Terao T, Ukita T (1968) Mercury compounds in the blood of rats treated with ethylmercuric chloride. Toxicol Appl Pharmacol 13: 165–173
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione. Anal Biochem 27: 502–522
Wood AJ, Viswalingam A, Glue P, Aronson JK, Grahame-Smith DG (1989) Measurement of cation transport in vivo in healthy volunteers after the oral administration of lithium carbonate. Clin Sci 76: 397–402
Wu G (1995a) Uptake of methylmercury cysteine by rat erythrocytes at lower temperature. J Appl Toxicol (in press)
Wu G (1995b) Methylmercury cysteine uptake by rat erythrocytes: evidence for several transport systems. J Appl Toxicol (in press)
Wu G (1995c) Discrimination of transport systems for methylmercury cysteine uptake in rat erythrocytes by inhibitors and other factors. Pharmacol Res 31: 195–203
Wu G (1995d) Effect of inhibitors and substrates on methyl mercury uptake by rat erythrocytes. Arch Toxicol (in press)
Wu G (1995e) Screen potential transport systems for methyl mercury uptake in rat erythrocytes at 5°, by using inhibitors and substrates. Pharmacol Toxicol 77: 169–176
Wu G, Hirayama K, Yasutake A (1994) Kinetic study on methylmercury uptake by erythrocytes. Jpn J Toxicol Environ Health 40: P-17
Yamaoka K, Nakagawa T, Uno T (1978) Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm 4: 165–175
Yasutake A, Hirayama K, Inoue M (1989) Mechanism of urinary excretion of methylmercury in mice. Arch Toxicol 63: 479–483
Yasutake A, Hirayama K, Inoue M (1990) Interaction of methylmercury compounds with albumin. Arch Toxicol 64: 639–643
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wu, G. Prediction of uptake of methyl mercury by rat erythrocytes using a two-compartment model. Arch Toxicol 70, 34–42 (1995). https://doi.org/10.1007/s002040050246
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s002040050246