Abstract
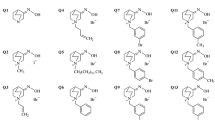
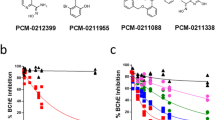
Six novel brominated bis-pyridinium oximes were designed and synthesized to increase their nucleophilicity and reactivation ability of phosphorylated acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Their pKa was valuably found lower to parent non-halogenated oximes. Stability tests showed that novel brominated oximes were stable in water, but the stability of di-brominated oximes was decreased in buffer solution and their degradation products were prepared and characterized. The reactivation screening of brominated oximes was tested on AChE and BChE inhibited by organophosphorus surrogates. Two mono-brominated oximes reactivated AChE comparably to non-halogenated analogues, which was further confirmed by reactivation kinetics. The acute toxicity of two selected brominated oximes was similar to commercially available oxime reactivators and the most promising brominated oxime was tested in vivo on sarin- and VX-poisoned rats. This brominated oxime showed interesting CNS distribution and significant reactivation effectiveness in blood. The same oxime resulted with the best protective index for VX-poisoned rats.









Similar content being viewed by others
Data availability
The raw experimental data from this study are available from the corresponding authors upon request.
References
Arnett EM, Reich R (1980) Electronic effects on the Menshutkin reaction. A complete kinetic and thermodynamic dissection of alkyl transfer to 3- and 4-substituted pyridines. J Am Chem Soc 102:5892–5902. https://doi.org/10.1021/ja00538a031
Bajgar J (2004) Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. In: Advances in Clinical Chemistry. Elsevier, pp 151–216
Čadež T, Kolić D, Šinko G, Kovarik Z (2021) Assessment of four organophosphorus pesticides as inhibitors of human acetylcholinesterase and butyrylcholinesterase. Sci Rep 11:21486. https://doi.org/10.1038/s41598-021-00953-9
Carletti E, Colletier J-P, Dupeux F et al (2010) Structural evidence that human acetylcholinesterase inhibited by tabun ages through o-dealkylation. J Med Chem 53:4002–4008. https://doi.org/10.1021/jm901853b
Clayden J, Greeves N, Warren S (2012) Organic chemistry, 2nd edn. OUP Oxford
Eichler T, Hauptmann S (2003) The chemistry of heterocycles: structures, reactions, synthesis, and applications, Wiley-VCH Verag GmbH&Co. KGaA
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Franjesevic AJ, Sillart SB, Beck JM et al (2019) Resurrection and reactivation of acetylcholinesterase and butyrylcholinesterase. Chem Eur J 25:5337–5371. https://doi.org/10.1002/chem.201805075
Gorecki L, Andrys R, Schmidt M et al (2020) Cysteine-targeted insecticides against A. gambiae acetylcholinesterase are neither selective nor reversible inhibitors. ACS Med Chem Lett 11:65–71. https://doi.org/10.1021/acsmedchemlett.9b00477
Gupta R (2015) Handbook of toxicology of chemical warfare agents, 2nd edn. Academic Press Elsevier, Amsterdam
Gupta B, Sharma R, Singh N et al (2013) In vitro reactivation kinetics of paraoxon- and DFP-inhibited electric eel AChE using mono- and bis-pyridinium oximes. Arch Toxicol https://doi.org/10.1007/s00204-013-1136-z
Howes L (2020) Novichok compound poisoned Navalny. C&EN Global Enterp 98:5–5. https://doi.org/10.1021/cen-09835-scicon3
John H, van der Schans MJ, Koller M et al (2018) Fatal sarin poisoning in Syria 2013: forensic verification within an international laboratory network. Forensic Toxicol 36:61–71. https://doi.org/10.1007/s11419-017-0376-7
Karasova JZ, Zemek F, Bajgar J et al (2011) Partition of bispyridinium oximes (trimedoxime and K074) administered in therapeutic doses into different parts of the rat brain. J Pharm Biomed Anal 54:1082–1087. https://doi.org/10.1016/j.jpba.2010.11.024
Karasova J, Zemek F, Musilek K, Kuca K (2012) Time-dependent changes of oxime K027 concentrations in different parts of rat central nervous system. Neurotox Res https://doi.org/10.1007/s12640-012-9329-4
Karasova JZ, Kvetina J, Tacheci I et al (2017a) Pharmacokinetic profile of promising acetylcholinesterase reactivators K027 and K203 in experimental pigs. Toxicol Lett 273:20–25. https://doi.org/10.1016/j.toxlet.2017.03.017
Karasova JZ, Maderycova Z, Tumova M et al (2017b) Activity of cholinesterases in a young and healthy middle-European population: relevance for toxicology, pharmacology and clinical praxis. Toxicol Lett 277:24–31. https://doi.org/10.1016/j.toxlet.2017.04.017
Katalinić M, Maček Hrvat N, Žďárová Karasová J et al (2015) Translation of in vitro to in vivo pyridinium oxime potential in tabun poisoning. Arh Hig Rada Toksikol 66:291–298. https://doi.org/10.1515/aiht-2015-66-2740
Kohoutova Z, Malinak D, Andrys R et al (2022) Charged pyridinium oximes with thiocarboxamide moiety are equally or less effective reactivators of organophosphate-inhibited cholinesterases compared to analogous carboxamides. J Enzyme Inhib Med Chem 37:760–767. https://doi.org/10.1080/14756366.2022.2041628
Kuca K, Bielavský J, Cabal J, Bielavská M (2003a) Synthesis of a potential reactivator of acetylcholinesterase—1-(4-hydroxyiminomethylpyridinium)-3-(carbamoylpyridinium) propane bromide. Tetrahedron Lett 44:3123–3125. https://doi.org/10.1016/S0040-4039(03)00538-0
Kuča K, Bielavský J, Cabal J, Kassa J (2003b) Synthesis of a new reactivator of tabun-inhibited acetylcholinesterase. Bioorg Med Chem Lett 13:3545–3547. https://doi.org/10.1016/S0960-894X(03)00751-0
Kuca K, Jun D, Bajgar J (2007) Currently used cholinesterase reactivators against nerve agent intoxication: comparison of their effectivity in vitro. Drug Chem Toxicol 30:31–40. https://doi.org/10.1080/01480540601017637
Lei C, Sun X (2018) Comparing lethal dose ratios using probit regression with arbitrary slopes. BMC Pharmacol Toxicol 19:61. https://doi.org/10.1186/s40360-018-0250-1
Meek E, Chambers H, Coban A et al (2012) Synthesis and in vitro and in vivo inhibition potencies of highly relevant nerve agent surrogates. Toxicol Sci 126:525–533. https://doi.org/10.1093/toxsci/kfs013
Millard CB, Kryger G, Ordentlich A et al (1999) Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level. Biochemistry 38:7032–7039. https://doi.org/10.1021/bi982678l
Misik J, Pavlikova R, Cabal J, Kuca K (2015) Acute toxicity of some nerve agents and pesticides in rats. Drug Chem Toxicol 38:32–36. https://doi.org/10.3109/01480545.2014.900070
Misik J, Nepovimova E, Pejchal J et al (2018) Cholinesterase inhibitor 6-chlorotacrine—in vivo toxicological profile and behavioural effects. Curr Alzheimer Res 15:552–560. https://doi.org/10.3109/01480545.2014.900070
Moshiri M, Darchini-Maragheh E, Balali-Mood M (2012) Advances in toxicology and medical treatment of chemical warfare nerve agents. Daru J Pharm Sci 20:81. https://doi.org/10.1186/2008-2231-20-81
Musil K, Florianova V, Bucek P et al (2016) Development and validation of a FIA/UV–vis method for pKa determination of oxime based acetylcholinesterase reactivators. J Pharm Biomed Anal 117:240–246. https://doi.org/10.1016/j.jpba.2015.09.010
Musilek K, Jun D, Cabal J et al (2007) Design of a potent reactivator of tabun-inhibited acetylcholinesterase–synthesis and evaluation of (E)-1-(4-carbamoylpyridinium)-4-(4-hydroxyiminomethylpyridinium)-but-2-ene dibromide (K203). J Med Chem 50:5514–5518. https://doi.org/10.1021/jm070653r
Musilek K, Malinak D, Nepovimova E et al (2020) Chapter 69—novel cholinesterase reactivators. In: Gupta RC (ed) Handbook of toxicology of chemical warfare agents, 3rd edn. Academic Press, Boston, pp 1161–1177
Nepovimova E, Kuca K (2018) Chemical warfare agent NOVICHOK—mini-review of available data. Food Chem Toxicol 121:343–350. https://doi.org/10.1016/j.fct.2018.09.015
Pejchal J, Novotný J, Mařák V et al (2012) Activation of p38 MAPK and expression of TGF-β1 in rat colon enterocytes after whole body γ-irradiation. Int J Radiat Biol 88:348–358. https://doi.org/10.3109/09553002.2012.654044
Quinn DM (1987) Acetylcholinesterase: enzyme structure, reaction dynamics, and virtual transition states. Chem Rev 87:955–979. https://doi.org/10.1021/cr00081a005
Saint-André G, Kliachyna M, Kodepelly S et al (2011) Design, synthesis and evaluation of new α-nucleophiles for the hydrolysis of organophosphorus nerve agents: application to the reactivation of phosphorylated acetylcholinesterase. Tetrahedron 67:6352–6361. https://doi.org/10.1016/j.tet.2011.05.130
Sakurada K, Matsubara K, Shimizu K et al (2003) Pralidoxime iodide (2-PAM) penetrates across the blood-brain barrier. Neurochem Res 28:1401–1407. https://doi.org/10.1023/a:1024960819430
Smith D, Anderson D, Degryse A-D et al (2018) Classification and reporting of severity experienced by animals used in scientific procedures: FELASA/ECLAM/ESLAV Working Group report. Lab Anim 52:5–57. https://doi.org/10.1177/0023677217744587
Tallarida RJ, Murray RB (1986) Manual of pharmacologic calculations. Springer, New York
Tambara K, Pantoş GD (2013) Conversion of aldoximes into nitriles and amides under mild conditions. Org Biomol Chem 11:2466–2472. https://doi.org/10.1039/C3OB27362H
Tu AT (1999) Overview of sarin terrorist attacks in Japan. In: Natural and selected synthetic toxins. J Am Chem Soc pp 304–317. https://doi.org/10.1021/bk-2000-0745.ch020
Vanova N, Hojna A, Pejchal J et al (2021) Determination of K869, a novel oxime reactivator of acetylcholinesterase, in rat body fluids and tissues by liquid-chromatography methods: pharmacokinetic study. J Pharm Sci 110:1842–1852. https://doi.org/10.1016/j.xphs.2021.01.031
Vega JA, Vaquero JJ, Alvarez-Builla J et al (1999) A new approach to the synthesis of 2-aminoimidazo[1,2-a]pyridine derivatives through microwave-assisted N-alkylation of 2-halopyridines. Tetrahedron 55:2317–2326. https://doi.org/10.1016/S0040-4020(99)00012-5
Watson A, Opresko D, Young RA et al (2015) Chapter 9—organophosphate nerve agents. In: Gupta RC (ed) Handbook of toxicology of chemical warfare agents, 2nd edn. Academic Press, Boston, pp 87–109
Worek F, Mast U, Kiderlen D et al (1999) Improved determination of acetylcholinesterase activity in human whole blood. Clin Chim Acta 288:73–90. https://doi.org/10.1016/S0009-8981(99)00144-8
Worek F, Thiermann H, Szinicz L, Eyer P (2004) Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem Pharmacol 68:2237–2248. https://doi.org/10.1016/j.bcp.2004.07.038
Worek F, von der Wellen J, Musilek K et al (2012) Reactivation kinetics of a homologous series of bispyridinium bis-oximes with nerve agent-inhibited human acetylcholinesterase. Arch Toxicol 86:1379–1386. https://doi.org/10.1007/s00204-012-0842-2
Žďárová Karasová J, Zemek F, Kassa J, Kuča K (2014) Entry of oxime K027 into the different parts of rat brain: comparison with obidoxime and oxime HI-6. J Appl Biomed 12:25–29. https://doi.org/10.1016/j.jab.2013.01.001
Zdarova Karasova J, Hepnarova V, Andrys R et al (2020a) Encapsulation of oxime K027 into cucurbit[7]uril: in vivo evaluation of safety, absorption, brain distribution and reactivation effectiveness. Toxicol Lett 320:64–72. https://doi.org/10.1016/j.toxlet.2019.11.021
Zdarova Karasova J, Mzik M, Kucera T et al (2020b) Interaction of Cucurbit[7]uril with oxime K027, atropine, and paraoxon: risky or advantageous delivery system? Int J Mol Sci 21:7883. https://doi.org/10.3390/ijms21217883
Zdarova Karasova J, Soukup O, Korabecny J et al (2021) Tacrine and its 7-methoxy derivate; time-change concentration in plasma and brain tissue and basic toxicological profile in rats. Drug Chem Toxicol 44:207–214. https://doi.org/10.1080/01480545.2019.1566350
Zorbaz T, Malinak D, Maraković N et al (2018) Pyridinium oximes with ortho-positioned chlorine moiety exhibit improved physicochemical properties and efficient reactivation of human acetylcholinesterase inhibited by several nerve agents. J Med Chem 61:10753–10766. https://doi.org/10.1021/acs.jmedchem.8b01398
Zorbaz T, Malinak D, Hofmanova T et al (2022) Halogen substituents enhance oxime nucleophilicity for reactivation of cholinesterases inhibited by nerve agents. Eur J Med Chem 238:114377. https://doi.org/10.1016/j.ejmech.2022.114377
Acknowledgements
This work was supported by the Czech Science Foundation (No. GA21-03000S) and University of Hradec Kralove (Faculty of Science, SV2112-2023). The authors are grateful to Ing. Veronika Santruckova and Bc. Radka Mikesova for their skillful technical assistance, and Dr. Jana Svobodova for NMR measurements. The graphical abstract was created with BioRender.com.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prchalova, E., Andrys, R., Pejchal, J. et al. Brominated oxime nucleophiles are efficiently reactivating cholinesterases inhibited by nerve agents. Arch Toxicol (2024). https://doi.org/10.1007/s00204-024-03791-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00204-024-03791-6