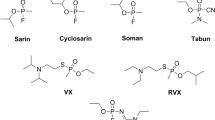
Abstract
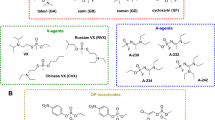
The misuse of novichok agents in assassination attempts has been reported in the international media since 2018. These relatively new class of neurotoxic agents is claimed to be more toxic than the agents of the G and V series and so far, there is no report yet in literature about potential antidotes against them. To shed some light into this issue, we report here the design and synthesis of NTMGMP, a surrogate of A-242 and also the first surrogate of a novichok agent useful for experimental evaluation of antidotes. Furthermore, the efficiency of the current commercial oximes to reactivate NTMGMP-inhibited acetylcholinesterase (AChE) was evaluated. The Ellman test was used to confirm the complete inhibition of AChE, and to compare the subsequent rates of reactivation in vitro as well as to evaluate aging. In parallel, molecular docking, molecular dynamics and MM-PBSA studies were performed on a computational model of the human AChE (HssAChE)/NTMGMP complex to assess the reactivation performances of the commercial oximes in silico. Experimental and theoretical studies matched the exact hierarchy of efficiency and pointed to trimedoxime as the most promising commercial oxime for reactivation of AChE inhibited by A-242.












Similar content being viewed by others
References
Abraham MJ, Murtola T, Schulz R et al (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25. https://doi.org/10.1016/j.softx.2015.06.001
Allgardsson A, Berg L, Akfur C et al (2016) Structure of a prereaction complex between the nerve agent sarin, its biological target acetylcholinesterase, and the antidote HI-6. Proc Natl Acad Sci 113(20):5514–5519
Bajgar J (2004) Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem 38(1):151–216
Bajgar J, Fusek J, Kuca K, Bartosova L, Jun D (2007) Treatment of organophosphate intoxication using cholinesterase reactivators: facts and fiction. Mini Rev Med Chem 7(5):461–466
Berman HM, Battistuz T, Bhat TN et al (2002) The protein data bank. Acta Crystallogr D Biol Crystallogr. https://doi.org/10.1107/S0907444902003451
Bester SM, Guelta MA, Cheung J et al (2018) Structural Insights of Stereospecific Inhibition of Human Acetylcholinesterase by VX and Subsequent Reactivation by HI-6. Chem Res Toxicol. https://doi.org/10.1021/acs.chemrestox.8b00294
Bolt HM, Hengstler JG (2022) Recent research on Novichok. Arch Toxicol 96:1137–1140
Bowie JU, Lüthy R, Eisenberg D (1991) A method to identify protein sequences that fold into a known three-dimensional stucture. Science. https://doi.org/10.1126/science.1853201
Bussi G, Donadio D, Parrinello M (2007) Canonical sampling through velocity rescaling. J Chem Phys. https://doi.org/10.1063/1.2408420
Cavalcante SF, Kitagawa DA, Rodrigues RB et al (2019a) One-pot synthesis of NEMP, a VX surrogate, and reactivation of NEMP-inhibited Electrophorus eel acetylcholinesterase by current antidotes. J Brazil Chem Soc 30:1095–1102
Cavalcante SFD, Kitagawa DAS, Rodrigues RB et al (2019b) Synthesis and in vitro evaluation of neutral aryloximes as reactivators of Electrophorus eel acetylcholinesterase inhibited by NEMP, a VX surrogate. Chem-Biol Interact. https://doi.org/10.1016/j.cbi.2019.05.048
da Silva JAV, Nepovimova E, Ramalho TC, Kuca K, Celmar Costa França T (2019) Molecular modeling studies on the interactions of 7-methoxytacrine-4-pyridinealdoxime, 4-PA, 2-PAM, and obidoxime with VX-inhibited human acetylcholinesterase: a near attack conformation approach. J Enzym Inhib Med Ch. https://doi.org/10.1080/14756366.2019.1609953
Da Silva AWS, Vranken WF (2012) ACPYPE-Antechamber python parser interface. BMC Res Notes 5(1):1–8
De A, Cavalcante SF, Kitagawa DAS, Rodrigues RB et al (2018) Straightforward, economical procedures for microscale ellman⇔s test for cholinesterase inhibition and reactivation. Quim Nova. https://doi.org/10.21577/0100-4042.20170278
de Cavalcante ASF, Simas AB, Kuča K (2019) Nerve agents’ surrogates: invaluable tools for development of acetylcholinesterase reactivators. Curr Organ Chem 23(14):1539–1559
Franca TCC, Kitagawa DAS, Cavalcante SFD, da Silva JAV, Nepovimova E, Kuca K (2019) Novichoks: the dangerous fourth generation of chemical weapons. Int J Mol Sci. https://doi.org/10.3390/ijms20051222
Goncalves AD, Franca TCC, Wilter A, Figueroa-Villar JD (2006) Molecular dynamics of the interaction of pralidoxime and deazapralidoxime with acetylcholinesterase inhibited by the neurotoxic agent tabun. J Brazil Chem Soc 17(5):968–975. https://doi.org/10.1590/s0103-50532006000500022
Goncalves AD, Franca TCC, Figueroa-Villar JD, Pascutti PG (2010) Conformational analysis of toxogonine, TMB-4 and HI-6 using PM6 and RM1 methods. J Brazil Chem Soc 21(1):179-U82. https://doi.org/10.1590/s0103-50532010000100025
Gorecki L, Korabecny J, Musilek K et al (2016) SAR study to find optimal cholinesterase reactivator against organophosphorous nerve agents and pesticides. Arch Toxicol 90(12):2831–2859
Hansen N, Van Gunsteren WF (2014) Practical aspects of free-energy calculations: a review. J Chem Theory Comput 10:2632–2647
Hehre WOSKPDBDA, Johnson JOP (2006) Spartan ‘08 Tutorial and User’s Guide. Wavefunction, Irvine
Homeyer N, Gohlke H (2012) Free energy calculations by the molecular mechanics poisson-boltzmann surface area method. Mol Inf. https://doi.org/10.1002/minf.201100135
Hosseini SE, Saeidian H, Amozadeh A, Naseri MT, Babri M (2016) Fragmentation pathways and structural characterization of organophosphorus compounds related to the Chemical Weapons Convention by electron ionization and electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. https://doi.org/10.1002/rcm.7757
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. Graphics 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Imrit YA, Bhakhoa H, Sergeieva T et al (2020) A theoretical study of the hydrolysis mechanism of A-234; the suspected novichok agent in the Skripal attack. RSC Adv 10(47):27884–27893. https://doi.org/10.1039/D0RA05086E
Jeong K, Choi J (2019) Theoretical study on the toxicity of ‘Novichok’ agent candidates. R Soc Open Sci. https://doi.org/10.1098/rsos.190414
Johnson LA, Robertson AJ, Baxter NJ et al (2018) Van der Waals contact between nucleophile and transferring phosphorus is insufficient to achieve enzyme transition-state architecture. ACS Catal. https://doi.org/10.1021/acscatal.8b01612
Jorgensen WL, Maxwell DS, Tirado-Rives J (1996) Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J Am Chem Soc. https://doi.org/10.1021/ja9621760
Kitagawa DA, Rodrigues RB, Silva TN et al (2021) Design, synthesis, in silico studies and in vitro evaluation of isatin-pyridine oximes hybrids as novel acetylcholinesterase reactivators. J Enzym Inhib Med Ch 36(1):1370–1377
Kloske M, Witkiewicz Z (2019) Novichoks—the A group of organophosphorus chemical warfare agents. Chemosphere 221:672–682. https://doi.org/10.1016/j.chemosphere.2019.01.054
Kontoyianni M, McClellan LM, Sokol GS (2004) Evaluation of docking performance: comparative data on docking algorithms. J Med Chem. https://doi.org/10.1021/jm0302997
Kua J, Zhang Y, Eslami AC, Butler JR, McCammon JA (2003) Studying the roles of W86, E202, and Y337 in binding of acetylcholine to acetylcholinesterase using a combined molecular dynamics and multiple docking approach. Protein Sci 12(12):2675–2684
Kucera T, Gorecki L, Soukup O et al (2019) Oxime K203: a drug candidate for the treatment of tabun intoxication. Arch Toxicol 93(3):673–691
Kumari R, Kumar R, Consortium OSDD, Lynn A (2014) g _ mmpbsa - A GROMACS tool for MM-PBSA and its optimization for high-throughput binding energy calculations. J Chem Inf Model. https://doi.org/10.1021/ci500020m
Lewis S (2018) Salisbury, Novichok and international law on the use of force. RUSI J 163(4):10–19. https://doi.org/10.1080/03071847.2018.1529889
Lüthy R, Bowie JU, Eisenberg D (1992) Assessment of protein models with three-dimensional profiles. Nature. https://doi.org/10.1038/356083a0
Malinak D, Nepovimova E, Jun D, Musilek K, Kuca K (2018) Novel group of AChE reactivators—synthesis, in vitro reactivation and molecular docking study. Molecules 23(9):2291
Meek EC, Chambers HW, Coban A et al (2012) Synthesis and in vitro and in vivo inhibition potencies of highly relevant nerve agent surrogates. Toxicol Sci. https://doi.org/10.1093/toxsci/kfs013
Mirzayanov VS (2009) State secrets: an insider`s chronicle of the Russian Chemical Weapons Program. Outskirts Press Inc, Parker
Nepovimova E, Kuca K (2018) Chemical warfare agent NOVICHOK—mini-review of available data. Food Chem Toxicol 121:343–350. https://doi.org/10.1016/j.fct.2018.09.015
Nepovimova E, Kuca K (2019) The history of poisoning: from ancient times until modern ERA. Arch Toxicol 93(1):11–24
Oh K-A, Yang GY, Jun D, Kuca K, Jung Y-S (2006) Bis-pyridiumaldoxime reactivators connected with CH2O (CH2) nOCH2 linkers between pyridinium rings and their reactivity against VX. Bioorg Med Chem Lett 16(18):4852–4855
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: A new molecular dynamics method. J Appl Phys. https://doi.org/10.1063/1.328693
Ramachandran GN, Ramakrishnan C, Sasisekharan V (1963) Stereochemistry of polypeptide chain configurations. J Mol Biol 7:95–99
Ribeiro AAST, Horta BAC, De Alencastro RB (2008) MKTOP: A program for automatic construction of molecular topologies. J Brazil Chem Soc. https://doi.org/10.1590/S0103-50532008000700031
Rocha GB, Freire RO, Simas AM, Stewart JJ (2006) Rm1: a reparameterization of am1 for h, c, n, o, p, s, f, cl, br, and i. J Comput Chem 27(10):1101–1111. https://doi.org/10.1002/jcc.20425
Santos MC, Botelho FD, Gonçalves AS, Kuca K, Nepovimova E, Cavalcante SFA, Lima ALS, França TCC (2022) Theoretical assessment of the performances of commercial oximes on the reactivation of acetylcholinesterase inhibited by the nerve agent A-242 (novichok). Food Chem Toxicol 165:113084. https://doi.org/10.1016/j.fct.2022.113084
Steindl D, Boehmerle W, Körner R et al (2021) Novichok nerve agent poisoning. Lancet 397(10270):249–252
Swain M (2012) Chemicalize. org. vol 52. ACS Publications, p 613–615
Tattersall JE (1993) Ion channel blockade by oximes and recovery of diaphragm muscle from soman poisoning in vitro. Br J Pharmacol 108(4):1006–1015
Thomsen R, Christensen MH (2006) MolDock: a new technique for high-accuracy molecular docking. J Med Chem 49(11):3315–3321. https://doi.org/10.1021/jm051197e
Walker RC, Crowley IF, Case DA (2008) The implementation of a fast and accurate QM/MM potential method in Amber. J Comput Chem. https://doi.org/10.1002/jcc.20857
Acknowledgements
The authors are grateful to the Military Institute of Engineering (IME), Institute of Chemical, Biological, Radiological and Nuclear Defense (IDQBRN), Brazilian Army Technological Center (CTEx), Federal University of Lavras (for software licenses), University of Hradec Kralové and the CQDM SynergiQc grant.
Funding
This research was funded by the Brazilian agencies Conselho Nacional de Pesquisa (CNPq), grant no. 308225/2018–0; Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), grant no. E-02/202.961/2017; IFES—PRPPG, grant number 10/2019 (Productivity Researcher Program PPP); and FAPES, grant number 03/2020-2020-WMT5F. The authors are also grateful to the Excelence project PrF UHK 2217/2022-2023 for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Santos, M.C., Botelho, F.D., Gonçalves, A.S. et al. Are the current commercially available oximes capable of reactivating acetylcholinesterase inhibited by the nerve agents of the A-series?. Arch Toxicol 96, 2559–2572 (2022). https://doi.org/10.1007/s00204-022-03316-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-022-03316-z