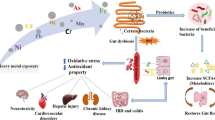
Abstract
Environmental chemicals such as inorganic arsenic (iAs) significantly contribute to redox toxicity in the human body by enhancing oxidative stress. Imbalanced oxidative stress rapidly interferes with gut homeostasis and affects variety of cellular processes such as proliferation, apoptosis, and maintenance of intestinal barrier integrity. It has been shown that gut microbiota are essential to protect against iAs3+-induced toxicity. However, the effect of microbial metabolites on iAs3+-induced toxicity and loss of gut barrier integrity has not been investigated. The objectives of the study are to investigate impact of iAs on gut barrier function and determine benefits of gut microbial metabolite, urolithin A (UroA) against iAs3+-induced adversaries on gut epithelium. We have utilized both colon epithelial cells and in a human intestinal 3D organoid model system to investigate iAs3+-induced cell toxicity, oxidative stress, and gut barrier dysfunction in the presence or absence of UroA. Here, we report that treatment with UroA attenuated iAs3+-induced cell toxicity, apoptosis, and oxidative stress in colon epithelial cells. Moreover, our data suggest that UroA significantly reduces iAs3+-induced gut barrier permeability and inflammatory markers in both colon epithelial cells and in a human intestinal 3D organoid model system. Mechanistically, UroA protected against iAs3+-induced disruption of tight junctional proteins in intestinal epithelial cells through blockade of oxidative stress and markers of inflammation. Taken together, our studies for the first time suggest that microbial metabolites such as UroA can potentially be used to protect against environmental hazards by reducing intestinal oxidative stress and by enhancing gut barrier function.
Highlights
-
The gut microbial metabolite Urolithin A (UroA) enhances cell viability during inorganic arsenic (iAs3+) exposure and prevents arsenite-induced cell death in colon epithelial cells.
-
UroA reduced iAs3+-induced oxidative stress in intestinal epithelial cells.
-
UroA alleviated iAs3+-induced barrier dysfunction in both colon epithelial cell monolayers and a human 3D small intestinal tissue model.
-
The protective effect of UroA is associated with enhanced accumulation of tight junctional proteins including Zonula occludens-1, Occludin, and Claudin-4 to repair iAs3+-induced intestinal barrier damage.










Similar content being viewed by others
Availability of data and materials
The data, analytic methods, and study materials will be made available to other researchers.
References
Agus A, Clement K, Sokol H (2020) Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 70:1174–1182
Andreux PA, Blanco-Bose W, Ryu D, Burdet F, Ibberson M, Aebischer P, Auwerx J, Singh A, Rinsch C (2019) The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat Metab 1(6):595–603
Antonelli R, Shao K, Thomas DJ, Sams R 2nd, Cowden J (2014) AS3MT, GSTO, and PNP polymorphisms: impact on arsenic methylation and implications for disease susceptibility. Environ Res 132:156–167
Au WY, Kwong YL (2008) Arsenic trioxide: safety issues and their management. Acta Pharmacol Sin 29(3):296–304
Aviello G, Knaus UG (2017) ROS in gastrointestinal inflammation: rescue or sabotage? Br J Pharmacol 174(12):1704–1718
Aviello G, Knaus UG (2018) NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol 11(4):1011–1023
Ayehunie S, Stevens Z, Landry T, Taimi M, Klausner M, Hayden P (2014) Novel 3D human small intestinal tissue model (EpiInestinal TM) to assess drug permeation & inflammation
Banerjee N, Banerjee M, Ganguly S, Bandyopadhyay S, Das JK, Bandyopadhay A, Chatterjee M, Giri AK (2008) Arsenic-induced mitochondrial instability leading to programmed cell death in the exposed individuals. Toxicology 246(2–3):101–111
Banerjee M, Ferragut Cardoso AP, Lykoudi A, Wilkey DW, Pan J, Watson WH, Garbett NC, Rai SN, Merchant ML, States JC (2020) Arsenite exposure displaces zinc from ZRANB2 leading to altered splicing. Chem Res Toxicol 33(6):1403–1417
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE (2014) Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 94(2):329–354
Biswas R, Ghosh P, Banerjee N, Das JK, Sau T, Banerjee A, Roy S, Ganguly S, Chatterjee M, Mukherjee A, Giri AK (2008) Analysis of T-cell proliferation and cytokine secretion in the individuals exposed to arsenic. Hum Exp Toxicol 27(5):381–386
Brabec JL, Wright J, Ly T, Wong HT, McClimans CJ, Tokarev V, Lamendella R, Sherchand S, Shrestha D, Uprety S, Dangol B, Tandukar S, Sherchand JB, Sherchan SP (2020) Arsenic disturbs the gut microbiome of individuals in a disadvantaged community in Nepal. Heliyon 6(1):e03313
Buckley A, Turner JR (2018) Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol 10(1):a029314–a029332
Calatayud M, Devesa V, Velez D (2013) Differential toxicity and gene expression in Caco-2 cells exposed to arsenic species. Toxicol Lett 218(1):70–80
Caricilli AM, Castoldi A, Câmara NO (2014) Intestinal barrier: a gentlemen’s agreement between microbiota and immunity. World J Gastrointest Pathophysiol 5(1):18–32
Carson RT, Koundouri P, Nauges C (2010) Arsenic mitigation in Bangladesh: a household labor market approach. Am J Agr Econ 93(2):407–414
Cerda B, Espin JC, Parra S, Martinez P, Tomas-Barberan FA (2004) The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur J Nutr 43(4):205–220
Cerda B, Periago P, Espin JC, Tomas-Barberan FA (2005a) Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds. J Agric Food Chem 53(14):5571–5576
Cerda B, Tomas-Barberan FA, Espin JC (2005b) Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability. J Agric Food Chem 53(2):227–235
Cervantes C, Ji G, Ramirez JL, Silver S (1994) Resistance to arsenic compounds in microorganisms. FEMS Microbiol Rev 15(4):355–367
Chi L, Bian X, Gao B, Tu P, Ru H, Lu K (2017) The effects of an environmentally relevant level of arsenic on the gut microbiome and its functional metagenome. Toxicol Sci 160(2):193–204
Chiocchetti GM, Vélez D, Devesa V (2018) Effect of subchronic exposure to inorganic arsenic on the structure and function of the intestinal epithelium. Toxicol Lett 286:80–88
Chiocchetti GM, Vélez D, Devesa V (2019a) Inorganic arsenic causes intestinal barrier disruption. Metallomics 11(8):1411–1418
Chiocchetti GM, Domene A, Kühl AA, Zúñiga M, Vélez D, Devesa V, Monedero V (2019b) In vivo evaluation of the effect of arsenite on the intestinal epithelium and associated microbiota in mice. Arch Toxicol 93(8):2127–2139
Choiniere J, Wang L (2016) Exposure to inorganic arsenic can lead to gut microbe perturbations and hepatocellular carcinoma. Acta Pharm Sin B 6(5):426–429
Claus SP, Guillou H, Ellero-Simatos S (2016a) The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes 2:16003
Coryell M, McAlpine M, Pinkham NV, McDermott TR, Walk ST (2018) The gut microbiome is required for full protection against acute arsenic toxicity in mouse models. Nat Commun 9(1):5424
Coryell M, Roggenbeck BA, Walk ST (2019) The human gut microbiome’s influence on arsenic toxicity. Curr Pharmacol Rep 5(6):491–504
Cui Y, Claus S, Schnell D, Runge F, MacLean C (2020) In-depth characterization of epiintestinal microtissue as a model for intestinal drug absorption and metabolism in human. Pharmaceutics 12(5):405–420
D’Amico D, Andreux PA, Valdés P, Singh A, Rinsch C, Auwerx J (2021) Impact of the natural compound urolithin A on health, disease, and aging. Trends Mol Med 27(7):687–699
Devriese S, Van den Bossche L, Van Welden S, Holvoet T, Pinheiro I, Hindryckx P, De Vos M, Laukens D (2017) T84 monolayers are superior to Caco-2 as a model system of colonocytes. Histochem Cell Biol 148(1):85–93
Espin JC, Gonzalez-Barrio R, Cerda B, Lopez-Bote C, Rey AI, Tomas-Barberan FA (2007) Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J Agric Food Chem 55(25):10476–10485
Espin JC, Larrosa M, Garcia-Conesa MT, Tomas-Barberan F (2013) Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: the evidence so far. Evid Based Complement Altern Med 2013:270418
Faita F, Cori L, Bianchi F, Andreassi MG (2013) Arsenic-induced genotoxicity and genetic susceptibility to arsenic-related pathologies. Int J Environ Res Public Health 10(4):1527–1546
Fernández Fernández N, Estevez Boullosa P, Gómez Rodríguez A, Rodríguez Prada JI (2019) A rare cause of gastric injury: arsenic intake. Am J Gastroenterol 114(8):1193
Flora SJS, Pachauri V (2013) Arsenic, free radical and oxidative stress. In: Kretsinger RH, Uversky VN, Permyakov EA (eds) Encyclopedia of metalloproteins. Springer, New York, pp 149–159
France MM, Turner JR (2017) The mucosal barrier at a glance. J Cell Sci 130(2):307–314
Garbinski LD, Rosen BP, Chen J (2019) Pathways of arsenic uptake and efflux. Environ Int 126:585–597
Garcia-Villalba R, Beltran D, Espin JC, Selma MV, Tomas-Barberan FA (2013) Time course production of urolithins from ellagic acid by human gut microbiota. J Agric Food Chem 61(37):8797–8806
Garza-Lombo C, Pappa A, Panayiotidis MI, Gonsebatt ME, Franco R (2019) Arsenic-induced neurotoxicity: a mechanistic appraisal. J Biol Inorg Chem 24(8):1305–1316
Ghosh S, Whitley CS, Haribabu B, Jala VR (2021) Regulation of intestinal barrier function by microbial metabolites. Cell Mol Gastroenterol Hepatol 11(5):1463–1482
Gonsebatt ME, Vega L, Montero R, Garcia-Vargas G, Del Razo LM, Albores A, Cebrian ME, Ostrosky-Wegman P (1994) Lymphocyte replicating ability in individuals exposed to arsenic via drinking water. Mutat Res 313(2–3):293–299
Gonsebatt ME, Vega L, Salazar AM, Montero R, Guzmán P, Blas J, Del Razo LM, García-Vargas G, Albores A, Cebrián ME, Kelsh M, Ostrosky-Wegman P (1997) Cytogenetic effects in human exposure to arsenic. Mutat Res 386(3):219–228
Gonzalez-Sarrias A, Gimenez-Bastida JA, Garcia-Conesa MT, Gomez-Sanchez MB, Garcia-Talavera NV, Gil-Izquierdo A, Sanchez-Alvarez C, Fontana-Compiano LO, Morga-Egea JP, Pastor-Quirante FA, Martinez-Diaz F, Tomas-Barberan FA, Espin JC (2010) Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol Nutr Food Res 54(3):311–322
Guha Mazumder D, Dasgupta UB (2011) Chronic arsenic toxicity: studies in West Bengal, India. Kaohsiung J Med Sci 27(9):360–370
Heilman J, Andreux P, Tran N, Rinsch C, Blanco-Bose W (2017) Safety assessment of urolithin A, a metabolite produced by the human gut microbiota upon dietary intake of plant derived ellagitannins and ellagic acid. Food Chem Toxicol 108(Pt A):289–297
Hong YS, Song KH, Chung JY (2014a) Health effects of chronic arsenic exposure. J Prev Med Public Health 47(5):245–252
Hossain M, Rahman SN, Bhattacharya P, Jacks G, Saha R, Rahman M (2015) Sustainability of arsenic mitigation interventions—an evaluation of different alternative safe drinking water options provided in Matlab, an arsenic hot spot in Bangladesh. Front Environ Sci 3:30
Hu Y, Li J, Lou B, Wu R, Wang G, Lu C, Wang H, Pi J, Xu Y (2020) The role of reactive oxygen species in arsenic toxicity. Biomolecules 10(2):240–270
Huang C, Ma WY, Li J, Dong Z (1999) Arsenic induces apoptosis through a c-Jun NH2-terminal kinase-dependent, p53-independent pathway. Cancer Res 59(13):3053–3058
Hunt KM, Srivastava RK, Elmets CA, Athar M (2014) The mechanistic basis of arsenicosis: pathogenesis of skin cancer. Cancer Lett 354(2):211–219
IARC (2012) Special report: policy, a review of human carcinogens—part c: metals, arsenic, dusts, and fibres. IARC Monogr Eval Carcinog Risks Hum 100:11–465
Jala VR, Bodduluri SR, Ghosh S, Chheda Z, Singh R, Smith ME, Chilton PM, Fleming CJ, Mathis SP, Sharma RK, Knight R, Yan J, Haribabu B (2021) Absence of CCR2 reduces spontaneous intestinal tumorigenesis in the Apc(Min) (/+) mouse model. Int J Cancer 148:2594–2607
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valko M (2011) Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31(2):95–107
Jovanovic P, Zoric L, Stefanovic I, Dzunic B, Djordjevic-Jocic J, Radenkovic M, Jovanovic M (2010) Lactate dehydrogenase and oxidative stress activity in primary open-angle glaucoma aqueous humour. Bosn J Basic Med Sci 10(1):83–88
Karim MR, Salam KA, Hossain E, Islam K, Ali N, Haque A, Saud ZA, Yeasmin T, Hossain M, Miyataka H, Himeno S, Hossain K (2010) Interaction between chronic arsenic exposure via drinking water and plasma lactate dehydrogenase activity. Sci Total Environ 409(2):278–283
Keim A, Rossler OG, Rothhaar TL, Thiel G (2012) Arsenite-induced apoptosis of human neuroblastoma cells requires p53 but occurs independently of c-Jun. Neuroscience 206:25–38
Kho ZY, Lal SK (2018) The human gut microbiome—a potential controller of wellness and disease. Front Microbiol 9:1835
Kim KB, Lee S, Kim JH (2020) Neuroprotective effects of urolithin A on H2O2-induced oxidative stress-mediated apoptosis in SK-N-MC cells. Nutr Res Pract 14(1):3–11
Koller BH, Snouwaert JN, Douillet C, Jania LA, El-Masri H, Thomas DJ, Stýblo M (2020) Arsenic metabolism in mice carrying a BORCS7/AS3MT locus humanized by syntenic replacement. Environ Health Perspect 128(8):87003
Larrosa M, Gonzalez-Sarrias A, Yanez-Gascon MJ, Selma MV, Azorin-Ortuno M, Toti S, Tomas-Barberan F, Dolara P, Espin JC (2010) Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem 21(8):717–725
Li D, Yang Y, Li Y, Li Z, Zhu X, Zeng X (2021a) Changes induced by chronic exposure to high arsenic concentrations in the intestine and its microenvironment. Toxicology 456:152767–152777
Li B, Lee C, Chuslip S, Lee D, Biouss G, Wu R, Koike Y, Miyake H, Ip W, Gonska T, Pierro A (2021b) Intestinal epithelial tight junctions and permeability can be rescued through the regulation of endoplasmic reticulum stress by amniotic fluid stem cells during necrotizing enterocolitis. FASEB J 35(1):e21265
Mazumder DN, Das Gupta J, Santra A, Pal A, Ghose A, Sarkar S (1998) Chronic arsenic toxicity in west Bengal—the worst calamity in the world. J Indian Med Assoc 96(1):4–7
Mazumder P, Sharma SK, Taki K, Kalamdhad AS, Kumar M (2020) Microbes involved in arsenic mobilization and respiration: a review on isolation, identification, isolates and implications. Environ Geochem Health 42(10):3443–3469
McDermott TR, Stolz JF, Oremland RS (2020) Arsenic and the gastrointestinal tract microbiome. Environ Microbiol Rep 12(2):136–159
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB (2014) Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20(7):1126–1167
Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA (2013) The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121(3):295–302
Nunez-Sanchez MA, Garcia-Villalba R, Monedero-Saiz T, Garcia-Talavera NV, Gomez-Sanchez MB, Sanchez-Alvarez C, Garcia-Albert AM, Rodriguez-Gil FJ, Ruiz-Marin M, Pastor-Quirante FA, Martinez-Diaz F, Yanez-Gascon MJ, Gonzalez-Sarrias A, Tomas-Barberan FA, Espin JC (2014) Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol Nutr Food Res 58(6):1199–1211
Odenwald MA, Turner JR (2013) Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol 11(9):1075–1083
Ohnishi K, Yoshida H, Shigeno K, Nakamura S, Fujisawa S, Naito K, Shinjo K, Fujita Y, Matsui H, Sahara N, Takeshita A, Satoh H, Terada H, Ohno R (2002) Arsenic trioxide therapy for relapsed or refractory Japanese patients with acute promyelocytic leukemia: need for careful electrocardiogram monitoring. Leukemia 16(4):617–622
Pan X, Jiang L, Zhong L, Geng C, Jia L, Liu S, Guan H, Yang G, Yao X, Piao F, Sun X (2016) Arsenic induces apoptosis by the lysosomal-mitochondrial pathway in INS-1 cells. Environ Toxicol 31(2):133–141
Pi J, Kumagai Y, Sun G, Yamauchi H, Yoshida T, Iso H, Endo A, Yu L, Yuki K, Miyauchi T, Shimojo N (2000) Decreased serum concentrations of nitric oxide metabolites among Chinese in an endemic area of chronic arsenic poisoning in inner Mongolia. Free Radic Biol Med 28(7):1137–1142
Podgorski J, Berg M (2020) Global threat of arsenic in groundwater. Science 368(6493):845–850
Prakash C, Chhikara S, Kumar V (2022) Mitochondrial dysfunction in arsenic-induced hepatotoxicity: pathogenic and therapeutic implications. Biol Trace Elem Res 200:261–270
Rao R (2008) Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front Biosci 13:7210–7226
Rao RK, Seth A, Sheth P (2004) Recent advances in alcoholic liver disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 286(6):G881–G884
Roggenbeck BA, Banerjee M, Leslie EM (2016) Cellular arsenic transport pathways in mammals. J Environ Sci (china) 49:38–58
Ryu D, Mouchiroud L, Andreux PA, Katsyuba E, Moullan N, Nicolet-Dit-Felix AA, Williams EG, Jha P, Lo Sasso G, Huzard D, Aebischer P, Sandi C, Rinsch C, Auwerx J (2016) Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med 22(8):879–888
Saha JC, Dikshit AK, Bandyopadhyay M, Saha KC (1999) A review of arsenic poisoning and its effects on human health. Crit Rev Environ Sci Technol 29(3):281–313
Saha P, Yeoh BS, Singh R, Chandrasekar B, Vemula PK, Haribabu B, Vijay-Kumar M, Jala VR (2016) Gut microbiota conversion of dietary ellagic acid into bioactive phytoceutical urolithin A inhibits heme peroxidases. PLoS One 11(6):e0156811
Sanyal T, Bhattacharjee P, Paul S, Bhattacharjee P (2020) Recent advances in arsenic research: significance of differential susceptibility and sustainable strategies for mitigation. Front Public Health 8:464
Satoh T, Enokido Y, Aoshima H, Uchiyama Y, Hatanaka H (1997) Changes in mitochondrial membrane potential during oxidative stress-induced apoptosis in PC12 cells. J Neurosci Res 50(3):413–420
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675
Schuhmacher-Wolz U, Dieter HH, Klein D, Schneider K (2009) Oral exposure to inorganic arsenic: evaluation of its carcinogenic and non-carcinogenic effects. Crit Rev Toxicol 39(4):271–298
Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D (2006) Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr 136(10):2481–2485
Seeram NP, Zhang Y, McKeever R, Henning SM, Lee RP, Suchard MA, Li Z, Chen S, Thames G, Zerlin A, Nguyen M, Wang D, Dreher M, Heber D (2008) Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects. J Med Food 11(2):390–394
Shao M, Zhu Y (2020) Long-term metal exposure changes gut microbiota of residents surrounding a mining and smelting area. Sci Rep 10(1):4453
Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY (1997) Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood 89(9):3354–3360
Singh R, Chandrashekharappa S, Bodduluri SR, Baby BV, Hegde B, Kotla NG, Hiwale AA, Saiyed T, Patel P, Vijay-Kumar M, Langille MGI, Douglas GM, Cheng X, Rouchka EC, Waigel SJ, Dryden GW, Alatassi H, Zhang HG, Haribabu B, Vemula PK, Jala VR (2019) Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun 10(1):89
Singh A, D'Amico D, Andreux PA, Dunngalvin G, Kern T, Blanco-Bose W, Auwerx J, Aebischer P, Rinsch C (2021) Direct supplementation with urolithin A overcomes limitations of dietary exposure and gut microbiome variability in healthy adults to achieve consistent levels across the population. Eur J Clin Nutr. https://doi.org/10.1038/s41430-021-00950-1
Ss DCR, Alava P, Zekker I, Du Laing G, Van de Wiele T (2014) Arsenic thiolation and the role of sulfate-reducing bacteria from the human intestinal tract. Environ Health Perspect 122(8):817–822
States JC, Barchowsky A, Cartwright IL, Reichard JF, Futscher BW, Lantz RC (2011) Arsenic toxicology: translating between experimental models and human pathology. Environ Health Perspect 119(10):1356–1363
Stevens JJ, Graham B, Walker AM, Tchounwou PB, Rogers C (2010) The effects of arsenic trioxide on DNA synthesis and genotoxicity in human colon cancer cells. Int J Environ Res Public Health 7(5):2018–2032
Tomas-Barberan FA, Garcia-Villalba R, Gonzalez-Sarrias A, Selma MV, Espin JC (2014) Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J Agric Food Chem 62(28):6535–6538
Tomas-Barberan FA, Gonzalez-Sarrias A, Garcia-Villalba R, Nunez-Sanchez MA, Selma MV, Garcia-Conesa MT, Espin JC (2016) Urolithins, the rescue of “old” metabolites to understand a “new” concept: metabotypes as a nexus between phenolic metabolism, microbiota dysbiosis and host health status. Mol Nutr Food Res 61:1500901
Tomás-Barberán FA, González-Sarrías A, García-Villalba R, Núñez-Sánchez MA, Selma MV, García-Conesa MT, Espín JC (2017) Urolithins, the rescue of “old” metabolites to understand a “new” concept: metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol Nutr Food Res 61(1):1500901–1500936
Tu P, Chi L, Bodnar W, Zhang Z, Gao B, Bian X, Stewart J, Fry R, Lu K (2020) Gut microbiome toxicity: connecting the environment and gut microbiome-associated diseases. Toxics 8(1):19
Vahter M (1999) Methylation of inorganic arsenic in different mammalian species and population groups. Sci Prog 82(Pt 1):69–88
Vahter M (2002) Mechanisms of arsenic biotransformation. Toxicology 181–182:211–217
Van de Wiele T, Gallawa CM, Kubachka KM, Creed JT, Basta N, Dayton EA, Whitacre S, Du Laing G, Bradham K (2010) Arsenic metabolism by human gut microbiota upon in vitro digestion of contaminated soils. Environ Health Perspect 118(7):1004–1009
Vantroyen B, Heilier JF, Meulemans A, Michels A, Buchet JP, Vanderschueren S, Haufroid V, Sabbe M (2004) Survival after a lethal dose of arsenic trioxide. J Toxicol Clin Toxicol 42(6):889–895
Weerasundara L, Ok YS, Bundschuh J (2021) Selective removal of arsenic in water: a critical review. Environ Pollut 268(Pt B):115668
Xu W, Zhang S, Jiang W, Xu S, Jin P (2020) Arsenic accumulation of realgar altered by disruption of gut microbiota in mice. Evid Based Complement Altern Med 2020:8380473
Zeisel MB, Dhawan P, Baumert TF (2019) Tight junction proteins in gastrointestinal and liver disease. Gut 68(3):547–561
Zhao RZ, Jiang S, Zhang L, Yu ZB (2019) Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med 44(1):3–15
Zheng Y (2017) Lessons learned from arsenic mitigation among private well households. Curr Environ Health Rep 4(3):373–382
Zuo L, Kuo WT, Turner JR (2020) Tight junctions as targets and effectors of mucosal immune homeostasis. Cell Mol Gastroenterol Hepatol 10(2):327–340
Acknowledgements
VRJ is supported by NIH/NCI (CA191683), NIH/NIGMS CoBRE Grant (P20GM125504-01), P30ES030283 (NIH/NIEHS), The Jewish Heritage Fund for Excellence Research Enhancement Grant, and JGBCC. The authors thank Dr. F.M. Ausubel for proof reading the manuscript and for insightful discussions. The authors thank Dr. Xu Jason from Integrated Toxicomics and Environmental Measurement Facility Core (ITEMFC), U of L for determining intra-cellular arsenic levels in cell lysates.
Funding
VRJ is supported by NIH/NCI (CA191683), NIH/NIGMS CoBRE Grant (P20GM125504-01), P30ES030283 (NIH/NIEHS), The Jewish Heritage Fund for Excellence Research Enhancement Grant, and UofL Health-BCC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
VRJ is one of the scientific co-founders of Artus Therapeutics. SG, MB, and BH have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghosh, S., Banerjee, M., Haribabu, B. et al. Urolithin A attenuates arsenic-induced gut barrier dysfunction. Arch Toxicol 96, 987–1007 (2022). https://doi.org/10.1007/s00204-022-03232-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-022-03232-2