Abstract
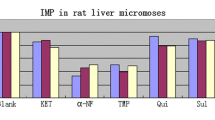
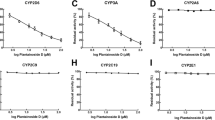
Sesquiterpene lactone helenalin is used as an antiphlogistic in European and Chinese folk medicine. The pharmacological activities of helenalin have been extensively investigated, yet insufficient information exists about its metabolic properties. The objectives of the present study were (1) to investigate the in vitro NADPH-dependent metabolism of helenalin (5 and 100 µM) using human and rat liver microsomes and liver cytosol, (2) to elucidate the role of human cytochrome P450 (CYP) enzymes in its oxidative metabolism, and (3) to study the inhibition of human CYPs by helenalin. Five oxidative metabolites were detected in NADPH-dependent human and rat liver microsomal incubations, while two reduced metabolites were detected only in NADPH-dependent human microsomal and cytosolic incubations. In human liver microsomes, the main oxidative metabolite was 14-hydroxyhelenalin, and in rat liver microsomes 9-hydroxyhelenalin. The overall oxidation of helenalin was several times more efficient in rat than in human liver microsomes. In humans, CYP3A4 and CYP3A5 followed by CYP2B6 were the main enzymes responsible for the hepatic metabolism of helenalin. The extrahepatic CYP2A13 oxidized helenalin most efficiently among CYP enzymes, possessing the Km value of 0.6 µM. Helenalin inhibited CYP3A4 (IC50 = 18.7 µM) and CYP3A5 (IC50 = 62.6 µM), and acted as a mechanism-based inhibitor of CYP2A13 (IC50 = 1.1 µM, KI = 6.7 µM, and kinact = 0.58 ln(%)/min). It may be concluded that the metabolism of helenalin differs between rats and humans, in the latter its oxidation is catalyzed by hepatic CYP2B6, CYP3A4, CYP3A5, and CYP3A7, and extrahepatic CYP2A13.





Similar content being viewed by others
References
Bergamante V, Ceschel GC, Marazzita S, Ronchi C, Fini A (2007) Effect of vehicles on topical application of aloe vera and arnica montana components. Drug Deliv 14(7):427–432. https://doi.org/10.1080/10717540701202960
Berges C, Fuchs D, Opelz G, Daniel V, Naujokat C (2009) Helenalin suppresses essential immune functions of activated CD4+ T cells by multiple mechanisms. Mol Immunol 46(15):2892–2901. https://doi.org/10.1016/j.molimm.2009.07.004
Boonruang S, Prakobsri K, Pouyfung P et al (2017) Inhibition of human cytochromes P450 2A6 and 2A13 by flavonoids, acetylenic thiophenes and sesquiterpene lactones from Pluchea indica and Vernonia cinerea. J Enzyme Inhib Med Chem 32(1):1136–1142. https://doi.org/10.1080/14756366.2017.1363741
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Buchele B, Zugmaier W, Lunov O, Syrovets T, Merfort I, Simmet T (2010) Surface plasmon resonance analysis of nuclear factor-kappaB protein interactions with the sesquiterpene lactone helenalin. Anal Biochem 401(1):30–37. https://doi.org/10.1016/j.ab.2010.02.020
Cai W, Zhang JY, Dong LY et al (2015) Identification of the metabolites of Ixerin Z from Ixeris sonchifolia Hance in rats by HPLC-LTQ-Orbitrap mass spectrometry. J Pharm Biomed Anal 107:290–297. https://doi.org/10.1016/j.jpba.2015.01.004
Chapman DE, Roberts GB, Reynolds DJ et al (1988) Acute toxicity of helenalin in BDF1 mice. Fundam Appl Toxicol 10(2):302–312
Chapman DE, Holbrook DJ, Chaney SG, Hall IH, Lee KH (1989) In vitro inhibition of mouse hepatic mixed-function oxidase enzymes by helenalin and alantolactone. Biochem Pharmacol 38(22):3913–3923. https://doi.org/10.1016/0006-2952(89)90668-0
Chapman DE, Holbrook DJ, Chaney SG, Hall IH, Lee KH (1991) In vivo and in vitro effects of helenalin on mouse hepatic microsomal cytochrome P450. Biochem Pharmacol 41(2):229–235. https://doi.org/10.1016/0006-2952(91)90481-j
Dirsch VM, Stuppner H, Vollmar AM (2001) Helenalin triggers a CD95 death receptor-independent apoptosis that is not affected by overexpression of Bcl-x(L) or Bcl-2. Cancer Res 61(15):5817–5823
Douglas JA, Smallfield BM, Burgess EJ et al (2004) Sesquiterpene lactones in Arnica montana: a rapid analytical method and the effects of flower maturity and simulated mechanical harvesting on quality and yield. Planta Med 70(2):166–170. https://doi.org/10.1055/s-2004-815495
Drogosz J, Janecka A (2019) Helenalin—a sesquiterpene lactone with multidirectional activity. Curr Drug Targets 20(4):444–452. https://doi.org/10.2174/1389450119666181012125230
European Medicines Agency (2014) Community herbal monograph on Arnica montana L., flos. EMA/HMPC/198793/2012. In: Committee on Herbal Medicinal Products (HMPC). https://www.ema.europa.eu/en/documents/herbal-monograph/final-community-herbal-monograph-arnica-montana-l-flos_en.pdf. Accessed 14 Sep 2021
Fayyaz A, Makwinja S, Auriola S, Raunio H, Juvonen RO (2018) Comparison of in vitro hepatic scoparone 7-O-demethylation between humans and experimental animals. Planta Med 84(5):320–328. https://doi.org/10.1055/s-0043-119886
Garcia-Pineres AJ, Castro V, Mora G et al (2001) Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J Biol Chem 276(43):39713–39720. https://doi.org/10.1074/jbc.M101985200
Hall IH, Lee KH, Mar EC, Starnes CO, Waddell TG (1977) Antitumor agents. 21. A proposed mechanism for inhibition of cancer growth by tenulin and helenalin and related cyclopentenones. J Med Chem 20(3):333–337. https://doi.org/10.1021/jm00213a003
Hall IH, Lee KH, Starnes CO et al (1979) Anti-inflammatory activity of sesquiterpene lactones and related compounds. J Pharm Sci 68(5):537–542. https://doi.org/10.1002/jps.2600680505
Hall IH, Lee KH, Starnes CO, Muraoka O, Sumida Y, Waddell TG (1980) Antihyperlipidemic activity of sesquiterpene lactones and related compounds. J Pharm Sci 69(6):694–697. https://doi.org/10.1002/jps.2600690622
Hempen C-H, Fischer T (2009) XIII—herbs that transform phlegm and stop coughing. In: Hempen C-H, Fischer T (eds) A materia medica for chinese medicine: plants, minerals, and animal products. Churchill, Livingstone, pp 618–679
Hoffmann R, von Schwarzenberg K, Lopez-Anton N et al (2011) Helenalin bypasses Bcl-2-mediated cell death resistance by inhibiting NF-kappaB and promoting reactive oxygen species generation. Biochem Pharmacol 82(5):453–463. https://doi.org/10.1016/j.bcp.2011.05.029
Huang PR, Yeh YM, Wang TC (2005) Potent inhibition of human telomerase by helenalin. Cancer Lett 227(2):169–174. https://doi.org/10.1016/j.canlet.2004.11.045
Itoh K, Yamamoto K, Adachi M, Kosaka T, Tanaka Y (2008) Leukotriene B4 12-hydroxydehydrogenase/15-ketoprostaglandin Delta 13-reductase (LTB4 12-HD/PGR) responsible for the reduction of a double-bond of the alpha, beta-unsaturated ketone of an aryl propionic acid non-steroidal anti-inflammatory agent CS-670. Xenobiotica 38(3):249–263. https://doi.org/10.1080/00498250701767667
Jang JH, Iqbal T, Min KJ et al (2013) Helenalin-induced apoptosis is dependent on production of reactive oxygen species and independent of induction of endoplasmic reticulum stress in renal cell carcinoma. Toxicol in Vitro 27(2):588–596. https://doi.org/10.1016/j.tiv.2012.10.014
Jodynis-Liebert J, Murias M, Bloszyk E (2000) Effect of sesquiterpene lactones on antioxidant enzymes and some drug-metabolizing enzymes in rat liver and kidney. Planta Med 66(3):199–205. https://doi.org/10.1055/s-2000-8566
Juvonen RO, Kuusisto M, Fohrgrup C et al (2016a) Inhibitory effects and oxidation of 6-methylcoumarin, 7-methylcoumarin and 7-formylcoumarin via human CYP2A6 and its mouse and pig orthologous enzymes. Xenobiotica 46(1):14–24. https://doi.org/10.3109/00498254.2015.1048327
Juvonen RO, Ahinko M, Huuskonen J, Raunio H, Pentikainen OT (2019a) Development of new Coumarin-based profluorescent substrates for human cytochrome P450 enzymes. Xenobiotica 49(9):1015–1024. https://doi.org/10.1080/00498254.2018.1530399
Juvonen RO, Novak F, Emmanouilidou E et al (2019b) Metabolism of scoparone in experimental animals and humans. Planta Med 85(6):453–464. https://doi.org/10.1055/a-0835-2301
Juvonen RO, Jokinen EM, Huuskonen J, Karkkainen O, Raunio H, Pentikainen OT (2021) Molecular docking and oxidation kinetics of 3-phenyl coumarin derivatives by human CYP2A13. Xenobiotica 2021:1–28. https://doi.org/10.1080/00498254.2021.1898700
Kitamura S, Kohno Y, Okamoto Y, Takeshita M, Ohta S (2002) Reductive metabolism of an alpha, beta-ketoalkyne, 4-phenyl-3-butyn-2-one, by rat liver preparations. Drug Metab Dispos 30(4):414–420. https://doi.org/10.1124/dmd.30.4.414
Kriplani P, Guarve K (2020) Recent Patents on Anti-Cancer Potential of Helenalin. Recent Pat Anticancer Drug Discov 15(2):132–142. https://doi.org/10.2174/1574892815666200702142601
Lang MA, Gielen JE, Nebert DW (1981) Genetic evidence for many unique liver microsomal P-450-mediated monooxygenase activities in heterogeneic stock mice. J Biol Chem 256(23):12068–12075
Lee KH (1973) Antitumor agents. V. Effect of epoxidation on cytotoxicity of helenalin-related derivatives. J Pharm Sci 62(6):1028–1029. https://doi.org/10.1002/jps.2600620645
Lee KH, Furukawa H (1972) Antitumor agents. 3. Synthesis and cytotoxic activity of helenalin amine adducts and related derivatives. J Med Chem 15(6):609–611. https://doi.org/10.1021/jm00276a010
Leven W, Willuhn G (1987) Sesquiterpene lactones from Arnica chamissonis less.: VI. Identification and quantitative determination by high-performance liquid and gas chromatography. J Chromatogr A 410:329–342
Li Y, Zeng Y, Huang Q et al (2019) Helenalin from Centipeda minima ameliorates acute hepatic injury by protecting mitochondria function, activating Nrf2 pathway and inhibiting NF-kappaB activation. Biomed Pharmacother 119:109435. https://doi.org/10.1016/j.biopha.2019.109435
Lim CB, Fu PY, Ky N et al (2012) NF-kappaB p65 repression by the sesquiterpene lactone, Helenalin, contributes to the induction of autophagy cell death. BMC Complement Altern Med 12:93. https://doi.org/10.1186/1472-6882-12-93
Lin X, Zhang S, Huang R et al (2014) Helenalin attenuates alcohol-induced hepatic fibrosis by enhancing ethanol metabolism, inhibiting oxidative stress and suppressing HSC activation. Fitoterapia 95:203–213. https://doi.org/10.1016/j.fitote.2014.03.020
Lyss G, Schmidt TJ, Merfort I, Pahl HL (1997) Helenalin, an anti-inflammatory sesquiterpene lactone from Arnica, selectively inhibits transcription factor NF-kappaB. Biol Chem 378(9):951–961. https://doi.org/10.1515/bchm.1997.378.9.951
Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I (1998) The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65. J Biol Chem 273(50):33508–33516. https://doi.org/10.1074/jbc.273.50.33508
Merrill JC, Kim HL, Safe S, Murray CA, Hayes MA (1988) Role of glutathione in the toxicity of the sesquiterpene lactones hymenoxon and helenalin. J Toxicol Environ Health 23(2):159–169. https://doi.org/10.1080/15287398809531103
Moore TW, Zhu S, Randolph R, Shoji M, Snyder JP (2014) Liver S9 fraction-derived metabolites of curcumin analogue UBS109. ACS Med Chem Lett 5(4):288–292. https://doi.org/10.1021/ml4002453
Moujir L, Callies O, Sousa P, Sharopov F, Seca AM (2020) Applications of sesquiterpene lactones: a review of some potential success cases. Appl Sci 10(9):3001
Niinivehmas S, Postila PA, Rauhamaki S et al (2018) Blocking oestradiol synthesis pathways with potent and selective coumarin derivatives. J Enzyme Inhib Med Chem 33(1):743–754. https://doi.org/10.1080/14756366.2018.1452919
Niwa T, Narita K, Okamoto A, Murayama N, Yamazaki H (2019) Comparison of steroid hormone hydroxylations by and docking to human cytochromes P450 3A4 and 3A5. J Pharm Pharm Sci 22(1):332–339. https://doi.org/10.18433/jpps30558
Niwa T, Okamoto A, Narita K et al (2020) Comparison of steroid hormone hydroxylation mediated by cytochrome P450 3A subfamilies. Arch Biochem Biophys 682:108283. https://doi.org/10.1016/j.abb.2020.108283
Peng Z, Wang Y, Gu X, Guo X, Yan C (2014) Study on the pharmacokinetics and metabolism of costunolide and dehydrocostus lactone in rats by HPLC-UV and UPLC-Q-TOF/MS. Biomed Chromatogr 28(10):1325–1334. https://doi.org/10.1002/bmc.3167
Rauhamaki S, Postila PA, Niinivehmas S et al (2018) Structure-activity relationship analysis of 3-phenylcoumarin-based monoamine oxidase B inhibitors. Front Chem 6:41. https://doi.org/10.3389/fchem.2018.00041
Schmidt TJ (1997) Helenanolide-type sesquiterpene lactones–III. Rates and stereochemistry in the reaction of helenalin and related helenanolides with sulfhydryl containing biomolecules. Bioorg Med Chem 5(4):645–653. https://doi.org/10.1016/s0968-0896(97)00003-5
Schmidt TJ (2000) Glutathione adducts of helenalin and 11 alpha,13-dihydrohelenalin acetate inhibit glutathione S-transferase from horse liver. Planta Med 66(2):106–109. https://doi.org/10.1055/s-2000-11123
Schmidt TJ, Lyss G, Pahl HL, Merfort I (1999) Helenanolide type sesquiterpene lactones. Part 5: the role of glutathione addition under physiological conditions. Bioorg Med Chem 7(12):2849–2855. https://doi.org/10.1016/s0968-0896(99)00234-5
Staneva J, Denkova P, Todorova M, Evstatieva L (2011) Quantitative analysis of sesquiterpene lactones in extract of Arnica montana L. by 1H NMR spectroscopy. J Pharm Biomed Anal 54(1):94–99. https://doi.org/10.1016/j.jpba.2010.08.018
Su T, Bao Z, Zhang QY, Smith TJ, Hong JY, Ding X (2000) Human cytochrome P450 CYP2A13: predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res 60(18):5074–5079
Todorova M, Trendafilova A, Vitkova A, Petrova M, Zayova E, Antonova D (2016) Developmental and environmental effects on sesquiterpene lactones in cultivated Arnica montana L. Chem Biodivers 13(8):976–981. https://doi.org/10.1002/cbdv.201500307
Tsai L, Highet RJ, Herz W (1969) The mass spectra of pseudoguaianolides related to helenalin. J Org Chem 34(4):945–948. https://doi.org/10.1021/jo01256a035
Wagner S, Merfort I (2007) Skin penetration behaviour of sesquiterpene lactones from different Arnica preparations using a validated GC-MSD method. J Pharm Biomed Anal 43(1):32–38. https://doi.org/10.1016/j.jpba.2006.06.008
Wagner S, Kratz F, Merfort I (2004a) In vitro behaviour of sesquiterpene lactones and sesquiterpene lactone-containing plant preparations in human blood, plasma and human serum albumin solutions. Planta Med 70(3):227–233. https://doi.org/10.1055/s-2004-815539
Wagner S, Suter A, Merfort I (2004b) Skin penetration studies of Arnica preparations and of their sesquiterpene lactones. Planta Med 70(10):897–903. https://doi.org/10.1055/s-2004-832613
Williams JA, Ring BJ, Cantrell VE et al (2002) Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab Dispos 30(8):883–891. https://doi.org/10.1124/dmd.30.8.883
Wu P, Su MX, Wang Y et al (2012) Supercritical fluid extraction assisted isolation of sesquiterpene lactones with antiproliferative effects from Centipeda minima. Phytochemistry 76:133–140. https://doi.org/10.1016/j.phytochem.2012.01.003
Yao D, Li Z, Huo C et al (2016) Identification of in vitro and in vivo metabolites of alantolactone by UPLC-TOF-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 1033–1034:250–260. https://doi.org/10.1016/j.jchromb.2016.08.034
Yu L, Jiang Y, Wang L, Sheng R, Hu Y, Zeng S (2013) Metabolism of BYZX in human liver microsomes and cytosol: identification of the metabolites and metabolic pathways of BYZX. PLoS ONE 8(3):e59882. https://doi.org/10.1371/journal.pone.0059882
Zanger UM, Schwab M (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138(1):103–141. https://doi.org/10.1016/j.pharmthera.2012.12.007
Zhang X, He J, Huang W et al (2018) Antiviral activity of the sesquiterpene lactones from centipeda minima against influenza a virus in vitro. Natural Prod Commun 13(2):00201
Zhou B, Ye J, Yang N et al (2018) Metabolism and pharmacokinetics of alantolactone and isoalantolactone in rats: Thiol conjugation as a potential metabolic pathway. J Chromatogr B Analyt Technol Biomed Life Sci 1072:370–378. https://doi.org/10.1016/j.jchromb.2017.11.039
Acknowledgements
We thank Hannele Jaatinen for technical assistance during the incubation experiments and Miia Reponen for technical assistance during the LC/MS analyses. We further thank Ivan Vokřál, Ph.D. for valuable comments and Daniel Paul Sampey for the English revision.
Funding
This work was funded by the Charles University Grant Agency (Project GAUK No. 1302120), the Czech Science Foundation (Grant no. 18-09946S), and Charles University (Research Project SVV 260 550). The School of Pharmacy mass spectrometry laboratory is supported by Biocenter Finland and Biocenter Kuopio.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MŠ, ROJ and SA. The first draft of the manuscript was written by MŠ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Human liver tissue was obtained from the University Hospital of Oulu (Oulu, Finland) as a surplus from cadaveric kidney transplantation donors. The procedure was approved by the Ethics Committee of the Medical Faculty of the University of Oulu (January 21, 1986). This study was performed in line with the principles of the Declaration of Helsinki. The Wistar rats used in this study were maintained according to European guidelines on the protection of animals used for scientific purposes (Directive 2010/63/EU). The animal experiments were approved by the Ethics Committee for Animal Experiments, University of Kuopio (Document 01-38, June 1, 2000).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Šadibolová, M., Juvonen, R.O., Auriola, S. et al. In vitro metabolism of helenalin and its inhibitory effect on human cytochrome P450 activity. Arch Toxicol 96, 793–808 (2022). https://doi.org/10.1007/s00204-021-03218-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-021-03218-6