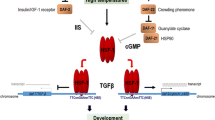
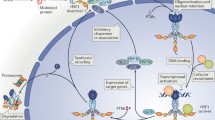
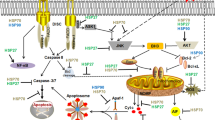
Abstract
Cells respond to protein-damaging (proteotoxic) stress by activation of the Heat Shock Response (HSR). The HSR provides cells with an enhanced ability to endure proteotoxic insults and plays a crucial role in determining subsequent cell death or survival. The HSR is, therefore, a critical factor that influences the toxicity of protein stress. While named for its vital role in the cellular response to heat stress, various components of the HSR system and the molecular chaperone network execute essential physiological functions as well as responses to other diverse toxic insults. The effector molecules of the HSR, the Heat Shock Factors (HSFs) and Heat Shock Proteins (HSPs), are also important regulatory targets in the progression of neurodegenerative diseases and cancers. Modulation of the HSR and/or its extended network have, therefore, become attractive treatment strategies for these diseases. Development of effective therapies will, however, require a detailed understanding of the HSR, important features of which continue to be uncovered and are yet to be completely understood. We review recently described and hallmark mechanistic principles of the HSR, the regulation and functions of HSPs, and contexts in which the HSR is activated and influences cell fate in response to various toxic conditions.






Similar content being viewed by others
References
Adelman K, Lis JT (2012) Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat Rev Genet 13(10):720–731
Alderson TR, Roche J, Gastall HY, Dias DM, Pritisanac I, Ying J, Bax A, Benesch JLP, Baldwin AJ (2019) Local unfolding of the HSP27 monomer regulates chaperone activity. Nat Commun 10(1):1068
Alford BD, Brandman O (2018) Quantification of Hsp90 availability reveals differential coupling to the heat shock response. J Cell Biol 217(11):3809–3816
Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, Piper PW, Prodromou C, Pearl LH (2006) Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature 440(7087):1013–1017
Alvira S, Cuellar J, Rohl A, Yamamoto S, Itoh H, Alfonso C, Rivas G, Buchner J, Valpuesta JM (2014) Structural characterization of the substrate transfer mechanism in Hsp70/Hsp90 folding machinery mediated by Hop. Nat Commun 5:5484
Amin V, Cumming DV, Latchman DS (1996) Over-expression of heat shock protein 70 protects neuronal cells against both thermal and ischaemic stress but with different efficiencies. Neurosci Lett 206(1):45–48
Ammirante M, Rosati A, Gentilella A, Festa M, Petrella A, Marzullo L, Pascale M, Belisario MA, Leone A, Turco MC (2008) The activity of hsp90 alpha promoter is regulated by NF-kappa B transcription factors. Oncogene 27(8):1175–1178
An JJ, Lee YP, Kim SY, Lee SH, Lee MJ, Jeong MS, Kim DW, Jang SH, Yoo KY, Won MH, Kang TC, Kwon OS, Cho SW, Lee KS, Park J, Eum WS, Choi SY (2008) Transduced human PEP-1-heat shock protein 27 efficiently protects against brain ischemic insult. FEBS J 275(6):1296–1308
Anckar J, Hietakangas V, Denessiouk K, Thiele DJ, Johnson MS, Sistonen L (2006) Inhibition of DNA binding by differential sumoylation of heat shock factors. Mol Cell Biol 26(3):955–964
Anfinsen CB (1973) Principles that govern the folding of protein chains. Science 181(4096):223–229
Arndt V, Dick N, Tawo R, Dreiseidler M, Wenzel D, Hesse M, Furst DO, Saftig P, Saint R, Fleischmann BK, Hoch M, Hohfeld J (2010) Chaperone-assisted selective autophagy is essential for muscle maintenance. Curr Biol 20(2):143–148
Arrigo AP, Gibert B (2012) HspB1 dynamic phospho-oligomeric structure dependent interactome as cancer therapeutic target. Curr Mol Med 12(9):1151–1163
Arrigo AP, Gibert B (2014) HspB1, HspB5 and HspB4 in human cancers: potent oncogenic role of some of their client proteins. Cancers (basel) 6(1):333–365
Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK (2000) HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6(4):435–442
Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK (2002) Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277(17):15028–15034
Bagatell R, Paine-Murrieta GD, Taylor CW, Pulcini EJ, Akinaga S, Benjamin IJ, Whitesell L (2000) Induction of a heat shock factor 1-dependent stress response alters the cytotoxic activity of hsp90-binding agents. Clin Cancer Res 6(8):3312–3318
Bai Y, Sosnick TR, Mayne L, Englander SW (1995) Protein folding intermediates: native-state hydrogen exchange. Science 269:192–197
Baird NA, Turnbull DW, Johnson EA (2006) Induction of the heat shock pathway during hypoxia requires regulation of heat shock factor by hypoxia-inducible factor-1. J Biol Chem 281(50):38675–38681
Balch WE, Morimoto RI, Dillin A, Kelly JW (2008b) Adapting proteostasis for disease intervention. Science 319(5865):916–919
Balch WE, Morimoto RI, Dillin A, Kelly JW (2008a) Adapting proteostasis for disease intervention. Science 319–919:916–919
Balchin D, Hayer-Hartl M, Hartl FU (2016) In vivo aspects of protein folding and quality control. Science 353(6294):aac4354
Baler R, Dahl G, Voellmy R (1993) Activation of human heat shock genes is accompanied by oligomerization, modification, and rapid translocation of heat shock transcription factor HSF1. Mol Cell Biol 13(4):2486–2496
Balogh G, Horvath I, Nagy E, Hoyk Z, Benko S, Bensaude O, Vigh L (2005) The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J 272(23):6077–6086
Becker J, Walter W, Yan W, Craig EA (1996) Functional interaction of cytosolic hsp70 and a DnaJ-related protein, Ydj1p, in protein translocation in vivo. Mol Cell Biol 16:4378–4386
Benbahouche Nel H, Iliopoulos I, Torok I, Marhold J, Henri J, Kajava AV, Farkas R, Kempf T, Schnolzer M, Meyer P, Kiss I, Bertrand E, Mechler BM, Pradet-Balade B (2014) Drosophila Spag is the homolog of RNA polymerase II-associated protein 3 (RPAP3) and recruits the heat shock proteins 70 and 90 (Hsp70 and Hsp90) during the assembly of cellular machineries. J Biol Chem 289(9):6236–6247
Bertelsen EB, Chang L, Gestwicki JE, Zuiderweg ERP (2009) Solution conformation of wild-type E. coli Hsp70 (DnaK) chaperone complexed with ADP and substrate. PNAS 106:8471–8476
Biebl MM, Buchner J (2019) Structure, function, and regulation of the Hsp90 machinery. Cold Spring Harb Perspect Biol 11(9):a034017
Biebl MM, Riedl M, Buchner J (2020) Hsp90 Co-chaperones Form Plastic Genetic Networks Adapted to Client Maturation. Cell Rep 32(8):108063
Blair LJ, Nordhues BA, Hill SE, Scaglione KM, O’Leary JC 3rd, Fontaine SN, Breydo L, Zhang B, Li P, Wang L, Cotman C, Paulson HL, Muschol M, Uversky VN, Klengel T, Binder EB, Kayed R, Golde TE, Berchtold N, Dickey CA (2013) Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J Clin Invest 123(10):4158–4169
Borges TJ, Murakami N, Machado FD, Murshid A, Lang BJ, Lopes RL, Bellan LM, Uehara M, Antunes KH, Perez-Saez MJ, Birrane G, Vianna P, Goncalves JIB, Zanin RF, Azzi J, Abdi R, Ishido S, Shin JS, Souza APD, Calderwood SK, Riella LV, Bonorino C (2018) March1-dependent modulation of donor MHC II on CD103(+) dendritic cells mitigates alloimmunity. Nat Commun 9(1):3482
Brandts JF, Hunt L (1966) The thermodynamics of protein denaturatio. III. The denaturation of ribonuclease in water and in aqueous urea and aqueous ethanol mixtures. J Am Chem Soc 89:4826–4838
Braselmann E, Chaney JL, Clark PL (2013) Folding the proteome. Trends Biochem Sci 38(7):337–344
Brehme M, Voisine C (2016) Model systems of protein-misfolding diseases reveal chaperone modifiers of proteotoxicity. Dis Model Mech 9(8):823–838
Brehme M, Voisine C, Rolland T, Wachi S, Soper JH, Zhu Y, Orton K, Villella A, Garza D, Vidal M, Ge H, Morimoto RI (2014) A chaperome subnetwork safeguards proteostasis in aging and neurodegenerative disease. Cell Rep 9(3):1135–1150
Brocchieri L, Conway de Macario E, Macario AJ (2008) hsp70 genes in the human genome: conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol Biol 8:19
Bruce JL, Price BD, Coleman CN, Calderwood SK (1993) Oxidative injury rapidly activates the heat shock transcription factor but fails to increase levels of heat shock proteins. Cancer Res 53(1):12–15
Bukau B, Horwich AL (1998) The Hsp70 and Hsp60 chaperone machines. Cell 92(3):351–366
Bunch H, Calderwood SK (2015) TRIM28 as a novel transcriptional elongation factor. BMC Mol Biol 16:14
Bunch H, Zheng X, Burkholder A, Dillon ST, Motola S, Birrane G, Ebmeier CC, Levine S, Fargo D, Hu G, Taatjes DJ, Calderwood SK (2014) TRIM28 regulates RNA polymerase II promoter-proximal pausing and pause release. Nat Struct Mol Biol 21(10):876–883
Bunch H, Lawney BP, Lin YF, Asaithamby A, Murshid A, Wang YE, Chen BP, Calderwood SK (2015) Transcriptional elongation requires DNA break-induced signalling. Nat Commun 6:10191
Burg MB, Ferraris JD, Dmitrieva NI (2007) Cellular response to hyperosmotic stresses. Physiol Rev 87(4):1441–1474
Cabrera M, Boronat S, Marte L, Vega M, Perez P, Ayte J, Hidalgo E (2020) Chaperone-facilitated aggregation of thermo-sensitive proteins shields them from degradation during heat stress. Cell Rep 30(7):2430-2443 e4
Cai Q, Ferraris JD, Burg MB (2004) Greater tolerance of renal medullary cells for a slow increase in osmolality is associated with enhanced expression of HSP70 and other osmoprotective genes. Am J Physiol Renal Physiol 286:F58–F67
Calderwood SK (2013) Molecular cochaperones: tumor growth and cancer treatment. Scientifica (cairo) 2013:217513
Calderwood SK (2018) Heat shock proteins and cancer: intracellular chaperones or extracellular signalling ligands? Philos Trans R Soc Lond B Biol Sci 373(1738):20160524
Calderwood SK, Gong J (2016) Heat shock proteins promote cancer: it’s a protection racket. Trends Biochem Sci 41(4):311–323
Calderwood SK, Neckers L (2016) Hsp90 in cancer: transcriptional roles in the nucleus. Adv Cancer Res 129:89–106
Calderwood SK, Murshid A, Prince T (2009) The shock of aging: molecular chaperones and the heat shock response in longevity and aging—a mini-review. Gerontology 55(5):550–558
Calderwood SK, Gong J, Murshid A (2016) Extracellular HSPs: the complicated roles of extracellular HSPs in immunity. Front Immunol 7:159
Cao SS, Kaufman RJ (2014) Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 21(3):396–413
Carper SW, Duffy JJ, Gerner EW (1987) Heat shock proteins in thermotolerance and other cellular processes. Cancer Res 47(20):5249–5255
Carra S, Alberti S, Benesch JLP, Boelens W, Buchner J, Carver JA, Cecconi C, Ecroyd H, Gusev N, Hightower LE, Klevit RE, Lee HO, Liberek K, Lockwood B, Poletti A, Timmerman V, Toth ME, Vierling E, Wu T, Tanguay RM (2019) Small heat shock proteins: multifaceted proteins with important implications for life. Cell Stress Chaperones 24(2):295–308
Caruccio L, Bae S, Liu AY, Chen KY (1997) The heat-shock transcription factor HSF1 is rapidly activated by either hyper- or hypo-osmotic stress in mammalian cells. Biochemical Journal 327:341–347
Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rebe C, Ghiringhelli F (2010) Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 120(2):457–471
Chang Y, Ostling P, Akerfelt M, Trouillet D, Rallu M, Gitton Y, El Fatimy R, Fardeau V, Le Crom S, Morange M, Sistonen L, Mezger V (2006) Role of heat-shock factor 2 in cerebral cortex formation and as a regulator of p35 expression. Genes Dev 20(7):836–847
Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N, Catley L, Tai YT, Hayashi T, Shringarpure R, Burger R, Munshi N, Ohtake Y, Saxena S, Anderson KC (2003) Hsp27 inhibits release of mitochondrial protein Smac in multiple myeloma cells and confers dexamethasone resistance. Blood 102(9):3379–3386
Chou SD, Prince T, Gong J, Calderwood SK (2012) mTOR is essential for the proteotoxic stress response, HSF1 activation and heat shock protein synthesis. PLoS ONE 7(6):e39679
Chu B, Zhong R, Soncin F, Stevenson MA, Calderwood SK (1998) Transcriptional activity of heat shock factor 1 at 37 degrees C is repressed through phosphorylation on two distinct serine residues by glycogen synthase kinase 3 and protein kinases Calpha and Czeta. J Biol Chem 273(29):18640–18646
Clerico EM, Tilitsky JM, Meng W, Gierasch LM (2015) How hsp70 molecular machines interact with their substrates to mediate diverse physiological functions. J Mol Biol 427(7):1575–1588
Cloutier P, Coulombe B (2013) Regulation of molecular chaperones through post-translational modifications: decrypting the chaperone code. Biochim Biophys Acta 1829(5):443–454
Colvin TA, Gabai VL, Gong J, Calderwood SK, Li H, Gummuluru S, Matchuk ON, Smirnova SG, Orlova NV, Zamulaeva IA, Garcia-Marcos M, Li X, Young ZT, Rauch JN, Gestwicki JE, Takayama S, Sherman MY (2014) Hsp70-Bag3 interactions regulate cancer-related signaling networks. Cancer Res 74(17):4731–4740
Conde R, Xavier J, McLoughlin C, Chinkers M, Ovsenek N (2005) Protein phosphatase 5 is a negative modulator of heat shock factor 1. J Biol Chem 280(32):28989–28996
Corcoran A, Cotter TG (2013) Redox regulation of protein kinases. FEBS J 280(9):1944–1965
Cox D, Whiten DR, Brown JWP, Horrocks MH, San Gil R, Dobson CM, Klenerman D, van Oijen AM, Ecroyd H (2018) The small heat shock protein Hsp27 binds alpha-synuclein fibrils, preventing elongation and cytotoxicity. J Biol Chem 293(12):4486–4497
Cuellar J, Ludlam WG, Tensmeyer NC, Aoba T, Dhavale M, Santiago C, Bueno-Carrasco MT, Mann MJ, Plimpton RL, Makaju A, Franklin S, Willardson BM, Valpuesta JM (2019) Structural and functional analysis of the role of the chaperonin CCT in mTOR complex assembly. Nat Commun 10(1):2865
Cunningham CN, Southworth DR, Krukenberg KA, Agard DA (2012) The conserved arginine 380 of Hsp90 is not a catalytic residue, but stabilizes the closed conformation required for ATP hydrolysis. Protein Sci 21(8):1162–1171
Dahl JU, Gray MJ, Jakob U (2015) Protein quality control under oxidative stress conditions. J Mol Biol 427(7):1549–1563
Davies MJ (2005) The oxidative environment and protein damage. Biochim Biophys Acta 1703(2):93–109
Del Razo LM, Quintanilla-Vega B, Brambila-Colombres E, Calderon-Aranda ES, Manno M, Albores A (2001) Stress proteins induced by arsenic. Toxicol Appl Pharmacol 177(2):132–148
Delaney JM (1990) Requirement of the Escherichia coli dnaK gene for thermotolerance and protection against H2O2. J Gen Microbiol 136(10):2113–2118
Delmas F, Schaak S, Gaubin Y, Croute F, Arrabit C, Murat JC (1998) Hsp72 mRNA production in cultured human cells submitted to nonlethal aggression by heat, ethanol, or propanol. Application to the detection of low concentraitons of chromium(VI) (potassium dichromate). Cell Biol Toxicol 14:39–46
Deshaies RJ, Koch BD, Werner-Washburne M, Craig EA, Schekman R (1988) A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature 332:800–805
Dewey WC, Diederich CJ, Dewhirst MW (2009) Hyperthermia classic commentary: ‘Arrhenius relationships from the molecule and cell to the clinic’ by William Dewey, Int J Hyperthermia, 10:457–483, 1994. Int J Hyperthermia 25(1):21–24
Dickson JA, Calderwood SK (1976) In vivo hyperthermia of Yoshida tumour induces entry of non-proliferating cells into cycle. Nature 263(5580):772–774
Dill KA, Ghosh K, Schmit JD (2011) Physical limits of cells and proteomes. Proc Natl Acad Sci USA 108(44):17876–17882
Dragovic Z, Broadley SA, Shomura Y, Bracher A, Hartl FU (2006) Molecular chaperones of the Hsp110 family act as nucleotide exchange factors of Hsp70s. EMBO J 25(11):2519–2528
Ecroyd H, Meehan S, Horwitz J, Aquilina JA, Benesch JL, Robinson CV, Macphee CE, Carver JA (2007) Mimicking phosphorylation of alphaB-crystallin affects its chaperone activity. Biochem J 401(1):129–141
Eguchi T, Sogawa C, Okusha Y, Uchibe K, Iinuma R, Ono K, Nakano K, Murakami J, Itoh M, Arai K, Fujiwara T, Namba Y, Murata Y, Ohyama K, Shimomura M, Okamura H, Takigawa M, Nakatsura T, Kozaki KI, Okamoto K, Calderwood SK (2018b) Organoids with cancer stem cell-like properties secrete exosomes and HSP90 in a 3D nanoenvironment. PLoS ONE 13(2):e0191109
Eguchi T, Lang BJ, Murshid A, Prince T, Gong J, Calderwood SK (2018a) Regulatory roles for Hsp70 in cancer incidence and tumor progression. Front Struct Biol 1:1–21
Eguchi T, Prince TL, Tran MT, Sogawa C, Lang BJ, Calderwood SK (2019) MZF1 and SCAND1 reciprocally regulate CDC37 gene expression in prostate cancer. Cancers (basel) 11(6):792
Ehrnsperger M, Graber S, Gaestel M, Buchner J (1997) Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO j 16:221–229
Ellis RJ (2007) Protein misassembly: macromolecular crowding and molecular chaperones. Adv Exp Med Biol 594:1–13
Ellis RJ, Hartl FU (1996) Protein folding in the cell: competing models of chaperonin function. FASEB J 10(1):20–26
Ellis RJ, van der Vies SM (1991) Molecular Chaperones. Annu Rev Biochem 60:321–347
Englander SW, Mayne L (2014) The nature of protein folding pathways. Proc Natl Acad Sci USA 111(45):15873–15880
Erdos G, Lee YJ (1994) Effect of staurosporine on the transcription of HSP70 heat shock gene in HT-29 cells. Biochem Biophys Res Commun 202(1):476–483
Fan F, Duan Y, Yang F, Trexler C, Wang H, Huang L, Li Y, Tang H, Wang G, Fang X, Liu J, Jia N, Chen J, Ouyang K (2020) Deletion of heat shock protein 60 in adult mouse cardiomyocytes perturbs mitochondrial protein homeostasis and causes heart failure. Cell Death Differ 27(2):587–600
Ferreira LMR, Cunha-Oliveira T, Sobral MC, Abreu PL, Alpoim MC, Urbano AM (2019) Impact of carcinogenic chromium on the cellular response to proteotoxic stress. Int J Mol Sci 20(19):4901
Fincato G, Polentarutti N, Sica A, Mantovani A, Colotta F (1991) Expression of a heat-inducible gene of the HSP70 family in human myelomonocytic cells: regulation by bacterial products and cytokines. Blood 77(3):579–586
Finka A, Goloubinoff P (2013) Proteomic data from human cell cultures refine mechanisms of chaperone-mediated protein homeostasis. Cell Stress Chaperones 18(5):591–605
Finka A, Mattoo RU, Goloubinoff P (2011) Meta-analysis of heat- and chemically upregulated chaperone genes in plant and human cells. Cell Stress Chaperones 16(1):15–31
Finka A, Sood V, Quadroni M, Rios Pde L, Goloubinoff P (2015) Quantitative proteomics of heat-treated human cells show an across-the-board mild depletion of housekeeping proteins to massively accumulate few HSPs. Cell Stress Chaperones 20(4):605–620
Fiorenza MT, Bevilacqua A, Canterini S, Torcia S, Pontecorvi M, Mangia F (2004) Early transcriptional activation of the hsp70.1 gene by osmotic stress in one-cell embryos of the mouse. Biol Reprod 70(6):1606–1613
Freilich R, Arhar T, Abrams JL, Gestwicki JE (2018) Protein-protein interactions in the molecular chaperone network. Acc Chem Res 51(4):940–949
Frydman J, Nimmesgern E, Erdjument-Bromage H, Wall JS, Tempst P, Hartl FU (1992) Function in protein folding of TRiC, a cytosolic ring complex containing TCP-1 and structurally related subunits. EMBO J 11(13):4767–4778
Fuhrmann-Stroissnigg H, Niedernhofer LJ, Robbins PD (2018) Hsp90 inhibitors as senolytic drugs to extend healthy aging. Cell Cycle 17(9):1048–1055
Galigniana MD, Erlejman AG, Monte M, Gomez-Sanchez C, Piwien-Pilipuk G (2010) The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events. Mol Cell Biol 30(5):1285–1298
Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G (2006) Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle 5(22):2592–2601
Gauthier MS, Cloutier P, Coulombe B (2018) Role of the PAQosome in regulating arrangement of protein quaternary structure in health and disease. Adv Exp Med Biol 1106:25–36
Gerner EW, Schneider MJ (1975) Induced thermal resistance in HeLa cells. Nature 256(5517):500–502
Gestaut D, Roh SH, Ma B, Pintilie G, Joachimiak LA, Leitner A, Walzthoeni T, Aebersold R, Chiu W, Frydman J (2019) The Chaperonin TRiC/CCT associates with prefoldin through a conserved electrostatic interface essential for cellular proteostasis. Cell 177(3):751-765 e15
Gething MJ (1996) Molecular chaperones: clasping the prize. Curr Biol 6(12):1573–1576
Grammatikakis N, Lin JH, Grammatikakis A, Tsichlis PN, Cochran BH (1999) p50(cdc37) acting in concert with Hsp90 is required for Raf-1 function. Mol Cell Biol 19(3):1661–1672
Grantham J (2020) The molecular chaperone CCT/TRiC: an essential component of proteostasis and a potential modulator of protein aggregation. Front Genet 11:172
Gray PJ Jr, Prince T, Cheng J, Stevenson MA, Calderwood SK (2008) Targeting the oncogene and kinome chaperone CDC37. Nat Rev Cancer 8(7):491–495
Green M, Schuetz TJ, Sullivan EK, Kingston RE (1995) A heat shock-responsive domain of human HSF1 that regulates transcription activation domain function. Mol Cell Biol 15(6):3354–3362
Guettouche T, Boellmann F, Lane WS, Voellmy R (2005) Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem 6:4
Guisbert E, Czyz DM, Richter K, McMullen PD, Morimoto RI (2013) Identification of a tissue-selective heat shock response regulatory network. PLoS Genet 9(4):e1003466
Gupta SC, Sharma A, Mishra M, Mishra RK, Chowdhuri DK (2010) Heat shock proteins in toxicology: how close and how far? Life Sci 86(11–12):377–384
Gupta A, Cooper ZA, Tulapurkar ME, Potla R, Maity T, Hasday JD, Singh IS (2013) Toll-like receptor agonists and febrile range hyperthermia synergize to induce heat shock protein 70 expression and extracellular release. J Biol Chem 288(4):2756–2766
Gutierres MBB, Bonorino CBC, Rigo MM (2020) ChaperISM: improved chaperone binding prediction using position-independent scoring matrices. Bioinformatics 36(3):735–741
Habtetsion T, Ding ZC, Pi W, Li T, Lu C, Chen T, Xi C, Spartz H, Liu K, Hao Z, Mivechi N, Huo Y, Blazar BR, Munn DH, Zhou G (2018) Alteration of tumor metabolism by CD4+ T cells leads to TNF-alpha-dependent intensification of oxidative stress and tumor cell death. Cell Metab 28(2):228-242 e6
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381(6583):571–579
Haslbeck M, Vierling E (2015) A first line of stress defense: small heat shock proteins and their function in protein homeostasis. J Mol Biol 427(7):1537–1548
Haug G, Leemhuis J, Tiemann D, Meyer DK, Aktories K, Barth H (2003) The host cell chaperone Hsp90 is essential for translocation of the binary Clostridium botulinum C2 toxin into the cytosol. J Biol Chem 278(34):32266–32274
He H, Soncin F, Grammatikakis N, Li Y, Siganou A, Gong J, Brown SA, Kingston RE, Calderwood SK (2003) Elevated expression of heat shock factor (HSF) 2A stimulates HSF1-induced transcription during stress. J Biol Chem 278(37):35465–35475
Hendrick JP, Hartl FU (1993) Molecular chaperone functions of heat shock proteins. Annu Rev Biochem 62:349–384
Hensen SM, Heldens L, van Enckevort CM, van Genesen ST, Pruijn GJ, Lubsen NH (2013) Activation of the antioxidant response in methionine deprived human cells results in an HSF1-independent increase in HSPA1A mRNA levels. Biochimie 95(6):1245–1251
Hernandez MP, Sullivan WP, Toft DO (2002) The assembly and intermolecular properties of the hsp70-Hop-hsp90 molecular chaperone complex. J Biol Chem 277(41):38294–38304
Hessling M, Richter K, Buchner J (2009) Dissection of the ATP-induced conformational cycle of the molecular chaperone Hsp90. Nat Struct Mol Biol 16(3):287–293
Hjerpe R, Bett JS, Keuss MJ, Solovyova A, McWilliams TG, Johnson C, Sahu I, Varghese J, Wood N, Wightman M, Osborne G, Bates GP, Glickman MH, Trost M, Knebel A, Marchesi F, Kurz T (2016) UBQLN2 mediates autophagy-independent protein aggregate clearance by the proteasome. Cell 166(4):935–949
Holland S, Lodwig E, Sideri T, Reader T, Clarke I, Gkargkas K, Hoyle DC, Delneri D, Oliver SG, Avery SV (2007) Application of the comprehensive set of heterozygous yeast deletion mutants to elucidate the molecular basis of cellular chromium toxicity. Genome Biol 8(12):R268
Houben B, Michiels E, Ramakers M, Konstantoulea K, Louros N, Verniers J, van der Kant R, De Vleeschouwer M, Chicoria N, Vanpoucke T, Gallardo R, Schymkowitz J, Rousseau F (2020) Autonomous aggregation suppression by acidic residues explains why chaperones favour basic residues. EMBO J 39(11):e102864
Hunt CR, Dix DJ, Sharma GG, Pandita RK, Gupta A, Funk M, Pandita TK (2004) Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice. Mol Cell Biol 24(2):899–911
Huot J, Roy G, Lambert H, Chretien P, Landry J (1991) Increased survival after treatments with anticancer agents of Chinese hamster cells expressing the human Mr 27,000 heat shock protein. Cancer Res 51(19):5245–5252
Huot J, Houle F, Spitz DR, Landry J (1996) HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Can Res 56:273–279
Inda MC, Joshi S, Wang T, Bolaender A, Gandu S, Koren Iii J, Che AY, Taldone T, Yan P, Sun W, Uddin M, Panchal P, Riolo M, Shah S, Barlas A, Xu K, Chan LYL, Gruzinova A, Kishinevsky S, Studer L, Fossati V, Noggle SA, White JR, de Stanchina E, Sequeira S, Anthoney KH, Steele JW, Manova-Todorova K, Patil S, Dunphy MP, Pillarsetty N, Pereira AC, Erdjument-Bromage H, Neubert TA, Rodina A, Ginsberg SD, De Marco Garcia N, Luo W, Chiosis G (2020) The epichaperome is a mediator of toxic hippocampal stress and leads to protein connectivity-based dysfunction. Nat Commun 11(1):319
Ito S, Nagata K (2017) Biology of Hsp47 (Serpin H1), a collagen-specific molecular chaperone. Semin Cell Dev Biol 62:142–151
Iwaki K, Chi SH, Dillmann WH, Mestril R (1993) Induction of HSP70 in cultured rat neonatal cardiomyocytes by hypoxia and metabolic stress. Circulation 87(6):2023–2032
Jaattela M, Saksela K, Saksela E (1989) Heat shock protects WEHI-164 target cells from the cytolysis by tumor necrosis factors alpha and beta. Eur J Immunol 19(8):1413–1417
Jaattela M, Wissing D, Bauer PA, Li GC (1992) Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J 11(10):3507–3512
Johnsson P, Ackley A, Vidarsdottir L, Lui WO, Corcoran M, Grander D, Morris KV (2013) A pseudogene long-noncoding-RNA network regulates PTEN transcription and translation in human cells. Nat Struct Mol Biol 20(4):440–446
Jornot L, Mirault ME, Junod AF (1991) Differential expression of hsp70 stress proteins in human endothelial cells exposed to heat shock and hydrogen peroxide. Am J Respir Cell Mol Biol 5(3):265–275
Kabani M, McLellan C, Raynes DA, Guerriero V, Brodsky JL (2002) HspBP1, a homologue of the yeast Fes1 and Sls1 proteins, is an Hsc70 nucleotide exchange factor. FEBS Lett 531:339–342
Kalinowska B, Banach M, Wisniowski Z, Konieczny L, Roterman I (2017) Is the hydrophobic core a universal structural element in proteins? J Mol Model 23(7):205
Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ (2003) A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425(6956):407–410
Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE (2009) Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 14(1):105–111
Kaushik S, Cuervo AM (2018) The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol 19(6):365–381
Kawazoe Y, Nakai A, Tanabe M, Nagata K (1998) Proteasome inhibition leads to the activation of all members of the heat-shock-factor family. Eur J Biochem 255(2):356–362
Kedersha N, Anderson P (2002) Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Transac 30(6):963–969
Kijima T, Prince TL, Tigue ML, Yim KH, Schwartz H, Beebe K, Lee S, Budzynski MA, Williams H, Trepel JB, Sistonen L, Calderwood S, Neckers L (2018) HSP90 inhibitors disrupt a transient HSP90-HSF1 interaction and identify a noncanonical model of HSP90-mediated HSF1 regulation. Sci Rep 8(1):6976
Kim SH, Kim JH, Erdos G, Lee YJ (1993) Effect of staurosporine on suppression of heat shock gene expression and thermotolerance development in HT-29 cells. Biochem Biophys Res Commun 193(2):759–763
King FW, Wawrzynow A, Hohfeld J, Zylicz M (2001) Co-chaperones Bag-1, Hop and Hsp40 regulate Hsc70 and Hsp90 interactions with wild-type or mutant p53. EMBO J 20(22):6297–6305
Kirschke E, Goswami D, Southworth D, Griffin PR, Agard DA (2014) Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell 157(7):1685–1697
Kirschke E, Roe-Zurz Z, Noddings C, Agard D (2020) The interplay between Bag-1, Hsp70, and Hsp90 reveals that inhibiting Hsp70 rebinding is essential for glucocorticoid receptor activity. bioRxiv. https://doi.org/10.1101/2020.05.03.075523
Kishinevsky S, Wang T, Rodina A, Chung SY, Xu C, Philip J, Taldone T, Joshi S, Alpaugh ML, Bolaender A, Gutbier S, Sandhu D, Fattahi F, Zimmer B, Shah SK, Chang E, Inda C, Koren J 3rd, Saurat NG, Leist M, Gross SS, Seshan VE, Klein C, Tomishima MJ, Erdjument-Bromage H, Neubert TA, Henrickson RC, Chiosis G, Studer L (2018) HSP90-incorporating chaperome networks as biosensor for disease-related pathways in patient-specific midbrain dopamine neurons. Nat Commun 9(1):4345
Kityk R, Kopp J, Sinning I, Mayer MP (2012) Structure and dynamics of the ATP-bound open conformation of Hsp70 chaperones. Mol Cell 48(6):863–874
Kityk R, Vogel M, Schlecht R, Bukau B, Mayer MP (2015) Pathways of allosteric regulation in Hsp70 chaperones. Nat Commun 6:8308
Kmiecik SW, Le Breton L, Mayer MP (2020) Feedback regulation of heat shock factor 1 (Hsf1) activity by Hsp70-mediated trimer unzipping and dissociation from DNA. EMBO J 39:104096
Koopman MB, Rudiger SG (2020) Behind closed gates - chaperones and charged residues determine protein fate. EMBO J 39(11):e104939
Koren J 3rd, Blagg BSJ (2020) The right tool for the job: an overview of Hsp90 inhibitors. Adv Exp Med Biol 1243:135–146
Koulov AV, LaPointe P, Lu B, Razvi A, Coppinger J, Dong MQ, Matteson J, Laister R, Arrowsmith C, Yates JR 3rd, Balch WE (2010) Biological and structural basis for Aha1 regulation of Hsp90 ATPase activity in maintaining proteostasis in the human disease cystic fibrosis. Mol Biol Cell 21(6):871–884
Krakowiak J, Zheng X, Patel N, Feder ZA, Anandhakumar J, Valerius K, Gross DS, Khalil AS, Pincus D (2018) Hsf1 and Hsp70 constitute a two-component feedback loop that regulates the yeast heat shock response. Elife 7:e31668
Kudla G, Helwak A, Lipinski L (2004) Gene conversion and GC-content evolution in mammalian Hsp70. Mol Biol Evol 21(7):1438–1444
Lancaster GI, Febbraio MA (2005) Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem 280(24):23349–23355
Lang BJ, Gorrell RJ, Tafreshi M, Hatakeyama M, Kwok T, Price JT (2016) The Helicobacter pylori cytotoxin CagA is essential for suppressing host heat shock protein expression. Cell Stress Chaperones 21(3):523–533
Lang BJ, Guerrero-Gimenez ME, Prince TL, Ackerman A, Bonorino C, Calderwood SK (2019) Heat shock proteins are essential components in transformation and tumor progression: cancer cell intrinsic pathways and beyond. Int J Mol Sci 20(18):4507
Laskey RA, Honda BM, Mills AD, Finch JT (1978) Nucleosomes are assembled by an acidic protein which binds histones and transfers them to DNA. Nature 275:416–420
Lee J, Seo J (2002) Differential expression of two stress-inducible hsp70 genes by various stressors. Exp Mol Med 34(2):131–136
Levinthal C (1968) Are there pathways for protein folding? Extrait Du J De Chimie Phys 65(1):44–45
Levinthal C (1969) How to fold graciously. Mössbaun Spectroscop Biol Syst Proc 67(41):22–24
Li GC, Fisher GA, Hahn GM (1982) Induction of thermotolerance and evidence for a well-defined, thermotropic cooperative process. Radiat Res 89(2):361–368
Li CY, Lee JS, Ko YG, Kim JI, Seo JS (2000) Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem 275(33):25665–25671
Li W, Li Y, Guan S, Fan J, Cheng CF, Bright AM, Chinn C, Chen M, Woodley DT (2007) Extracellular heat shock protein-90alpha: linking hypoxia to skin cell motility and wound healing. EMBO J 26(5):1221–1233
Li Z, Hartl FU, Bracher A (2013a) Structure and function of hip, an attenuator of the Hsp70 chaperone cycle. Nat Struct Mol Biol 20(8):929–935
Li W, Tsen F, Sahu D, Bhatia A, Chen M, Multhoff G, Woodley DT (2013b) Extracellular Hsp90 (eHsp90) as the actual target in clinical trials: intentionally or unintentionally. Int Rev Cell Mol Biol 303:203–235
Li H, Zhu H, Sarbeng EB, Liu Q, Tian X, Yang Y, Lyons C, Zhou L, Liu Q (2020) An unexpected second binding site for polypeptide substrates is essential for Hsp70 chaperone activity. J Biol Chem 295(2):584–596
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677
Lis JT, Mason P, Peng J, Price DH, Werner J (2000) P-TEFb kinase recruitment and function at heat shock loci. Genes Dev 14(7):792–803
Liu Y, Yang Q, Zhao F (2021) Synonymous but not silent: the codon usage code for gene expression and protein folding. Annu Rev Biochem. https://doi.org/10.1146/annurev-biochem-071320-112701
Luo W, Dou F, Rodina A, Chip S, Kim J, Zhao Q, Moulick K, Aguirre J, Wu N, Greengard P, Chiosis G (2007) Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc Natl Acad Sci USA 104(22):9511–9516
Ma Y, Hendershot LM (2004) ER chaperone functions during normal and stress conditions. J Chem Neuroanat 28(1–2):51–65
Mambula SS, Calderwood SK (2006) Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol 177(11):7849–7857
Mambula SS, Stevenson MA, Ogawa K, Calderwood SK (2007) Mechanisms for Hsp70 secretion: crossing membranes without a leader. Methods 43(3):168–175
Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH (1995) Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest 95(4):1446–1456
Masser AE, Kang W, Roy J, Mohanakrishnan Kaimal J, Quintana-Cordero J, Friedlander MR, Andreasson C (2019) Cytoplasmic protein misfolding titrates Hsp70 to activate nuclear Hsf1. Elife 8:047791
Mathew A, Mathur SK, Morimoto RI (1998) Heat shock response and protein degradation: regulation of HSF2 by the ubiquitin-proteasome pathway. Mol Cell Biol 18(9):5091–5098
McCallister C, Siracusa MC, Shirazi F, Chalkia D, Nikolaidis N (2015) Functional diversification and specialization of cytosolic 70-kDa heat shock proteins. Sci Rep 5:9363
McDuffee AT, Senisterra G, Huntley S, Lepock JR, Sekhar KR, Meredith MJ, Borrelli MJ, Morrow JD, Freeman ML (1997) Proteins containing non-native disulfide bonds generated by oxidative stress can act as signals for the induction of the heat shock response. J Cell Physiol 171:143–151
McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ (1998) Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. PNAS 273(13):7523–7528
Mehlen P, Preville X, Chareyron P, Briolay J, Klemenz R, Arrigo AP (1995) Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol 154(1):363–374
Meng W, Clerico EM, McArthur N, Gierasch LM (2018) Allosteric landscapes of eukaryotic cytoplasmic Hsp70s are shaped by evolutionary tuning of key interfaces. Proc Natl Acad Sci U S A 115(47):11970–11975
Mogk A, Bukau B, Kampinga HH (2018) Cellular handling of protein aggregates by disaggregation machines. Mol Cell 69(2):214–226
Mollapour M, Tsutsumi S, Truman AW, Xu W, Vaughan CK, Beebe K, Konstantinova A, Vourganti S, Panaretou B, Piper PW, Trepel JB, Prodromou C, Pearl LH, Neckers L (2011b) Threonine 22 phosphorylation attenuates Hsp90 interaction with cochaperones and affects its chaperone activity. Mol Cell 41(6):672–681
Mollapour M, Tsutsumi S, Kim YS, Trepel J, Neckers L (2011a) Casein kinase 2 phosphorylation of Hsp90 threonine 22 modulates chaperone function and drug sensitivity. Oncotarget 2(5):407–417
Moran Luengo T, Kityk R, Mayer MP, Rudiger SGD (2018) Hsp90 breaks the deadlock of the Hsp70 chaperone system. Mol Cell 70(3):545-552 e9
Moran Luengo T, Mayer MP, Rudiger SGD (2019) The Hsp70-Hsp90 chaperone cascade in protein folding. Trends Cell Biol 29(2):164–177
Morgner N, Schmidt C, Beilsten-Edmands V, Ebong IO, Patel NA, Clerico EM, Kirschke E, Daturpalli S, Jackson SE, Agard D, Robinson CV (2015) Hsp70 forms antiparallel dimers stabilized by post-translational modifications to position clients for transfer to Hsp90. Cell Rep 11(5):759–769
Mosser DD, Theodorakis NG, Morimoto RI (1988) Coordinate changes in heat shock element-binding activity and HSP70 gene transcription rates in human cells. Mol Cell Biol 8(11):4736–4744
Motlagh HN, Wrabl JO, Li J, Hilser VJ (2014) The ensemble nature of allostery. Nature 508(7496):331–339
Muller P, Ruckova E, Halada P, Coates PJ, Hrstka R, Lane DP, Vojtesek B (2013) C-terminal phosphorylation of Hsp70 and Hsp90 regulates alternate binding to co-chaperones CHIP and HOP to determine cellular protein folding/degradation balances. Oncogene 32(25):3101–3110
Munoz-Hernandez H, Pal M, Rodriguez CF, Fernandez-Leiro R, Prodromou C, Pearl LH, Llorca O (2019) Structural mechanism for regulation of the AAA-ATPases RUVBL1-RUVBL2 in the R2TP co-chaperone revealed by cryo-EM. Sci Adv 5(5):eaaw1616
Murshid A, Gong J, Calderwood SK (2010) Heat shock protein 90 mediates efficient antigen cross presentation through the scavenger receptor expressed by endothelial cells-I. J Immunol 185(5):2903–2917
Murshid A, Borges TJ, Bonorino C, Lang BJ, Calderwood SK (2019) Immunological outcomes mediated upon binding of heat shock proteins to scavenger receptors SCARF1 and LOX-1, and endocytosis by mononuclear phagocytes. Front Immunol 10:3035
Nagy E, Balogi Z, Gombos I, Akerfelt M, Bjorkbom A, Balogh G, Torok Z, Maslyanko A, Fiszer-Kierzkowska A, Lisowska K, Slotte PJ, Sistonene L, Horvath I, Vigh L (2007) Hyperfluidization-coupled membrane microdomain reorganization is linked to activation of the heat shock response in a murine melanoma cell line. PNAS 104(19):7945–7950
Nakai A (2016) Molecular basis of HSF regulation. Nat Struct Mol Biol 23(2):93–95
Narasimhan P, Swanson RA, Sagar SM, Sharp FR (1996) Astrocyte survival and HSP70 heat shock protein induction following heat shock and acidosis. Glia 17:147–159
Neef DW, Jaeger AM, Gomez-Pastor R, Willmund F, Frydman J, Thiele DJ (2014) A direct regulatory interaction between chaperonin TRiC and stress-responsive transcription factor HSF1. Cell Rep 9(3):955–966
Newton EM, Knauf U, Green M, Kingston RE (1996) The regulatory domain of human heat shock factor 1 is sufficient to sense heat stress. Mol Cell Biol 16(3):839–846
Nitika, Truman AW (2017) Cracking the chaperone code: cellular roles for Hsp70 phosphorylation. Trends Biochem Sci 42(12):932–935
Noddings CM, Wang RY, Agard DA (2020) GR chaperone cycle mechanism revealed by cryo-EM: reactivation of GR by the GR:Hsp90:p23 client-maturation complex. bioRxiv. https://doi.org/10.1101/2020.09.12.294975
Nollen EA, Kabakov AE, Brunsting JF, Kanon B, Hohfeld J, Kampinga HH (2001) Modulation of in vivo HSP70 chaperone activity by Hip and Bag-1. J Biol Chem 276(7):4677–4682
O’Brien D, Jones LM, Good S, Miles J, Vijayabaskar MS, Aston R, Smith CE, Westhead DR, van Oosten-Hawle P (2018) A PQM-1-mediated response triggers transcellular chaperone signaling and regulates organismal proteostasis. Cell Rep 23(13):3905–3919
Obrador E, Navarro J, Mompo J, Asensi M, Pellicer JA, Estrela JM (1998) Regulation of tumour cell sensitivity to TNF-induced oxidative stress and cytotoxicity: role of glutathione. BioFactors 8:23–36
O’Brien D, van Oosten-Hawle P (2016) Regulation of cell-non-autonomous proteostasis in metazoans. Essays Biochem 60(2):133–142
Oh HJ, Chen X, Subjeck JR (1997) Hsp110 protects heat-denatured proteins and confers cellular thermoresistance. J Biol Chem 272(50):31636–31640
Orosz A, Wisniewski J, Wu C (1996) Regulation of Drosophila heat shock factor trimerization: global sequence requirements and independence of nuclear localization. Mol Cell Biol 16(12):7018–7030
Paci A, Liu XH, Huang H, Lim A, Houry WA, Zhao R (2012) The stability of the small nucleolar ribonucleoprotein (snoRNP) assembly protein Pih1 in Saccharomyces cerevisiae is modulated by its C terminus. J Biol Chem 287(52):43205–43214
Park JM, Werner J, Kim JM, Lis JT, Kim YJ (2001) Mediator, not holoenzyme, is directly recruited to the heat shock promoter by HSF upon heat shock. Mol Cell 8(1):9–19
Patinen T, Adinolfi S, Cortes CC, Harkonen J, Jawahar Deen A, Levonen AL (2019) Regulation of stress signaling pathways by protein lipoxidation. Redox Biol 23:101114
Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP (2002) Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol 22(3):816–834
Perotti C, Liu R, Parusel CT, Bocher N, Schultz J, Bork P, Pfitzner E, Groner B, Shemanko CS (2008) Heat shock protein-90-alpha, a prolactin-STAT5 target gene identified in breast cancer cells, is involved in apoptosis regulation. Breast Cancer Res 10(6):R94
Pignataro L, Miller AN, Ma L, Midha S, Protiva P, Herrera DG, Harrison NL (2007) Alcohol regulates gene expression in neurons via activation of heat shock factor 1. J Neurosci 27(47):12957–12966
Pockley AG (2002) Heat shock proteins, inflammation, and cardiovascular disease. Circulation 105(8):1012–1017
Pockley AG, De Faire U, Kiessling R, Lemne C, Thulin T, Frostegard J (2002) Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens 20(9):1815–1820
Price BD, Calderwood SK (1991) Ca2+ Is essential for multistep activation of the heat shock factor in permeabilized cells. Mol Cell Biol 11(6):3365–3368
Prince T, Matts RL (2004) Definition of protein kinase sequence motifs that trigger high affinity binding of Hsp90 and Cdc37. J Biol Chem 279(38):39975–39981
Prince TL, Kijima T, Tatokoro M, Lee S, Tsutsumi S, Yim K, Rivas C, Alarcon S, Schwartz H, Khamit-Kush K, Scroggins BT, Beebe K, Trepel JB, Neckers L (2015) Client proteins and small molecule inhibitors display distinct binding preferences for constitutive and stress-induced HSP90 isoforms and their conformationally restricted mutants. PLoS ONE 10(10):e0141786
Prince T, Ackerman A, Cavanaugh A, Schreiter B, Juengst B, Andolino C, Danella J, Chernin M, Williams H (2018) Dual targeting of HSP70 does not induce the heat shock response and synergistically reduces cell viability in muscle invasive bladder cancer. Oncotarget 9(66):32702–32717
Prince TL, Lang BJ, Guerrero-Gimenez ME, Fernandez-Munoz JM, Ackerman A, Calderwood SK (2020) HSF1: primary factor in molecular chaperone expression and a major contributor to cancer morbidity. Cells 9(4):1046
Prodromou C, Panaretou B, Chohan S, Siligardi G, O’Brien R, Ladbury JE, Roe SM, Piper PW, Pearl LH (2000) The ATPase cycle of Hsp90 drives a molecular ‘clamp’ via transient dimerization of the N-terminal domains. EMBO J 19(16):4383–4392
Qian SB, McDonough H, Boellmann F, Cyr DM, Patterson C (2006) CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature 440(7083):551–555
Rabindran SK, Haroun RI, Clos J, Wisniewski J, Wu C (1993) Regulation of heat shock factor trimer formation: role of a conserved leucine zipper. Science 259(5092):230–234
Rao J, Lee P, Benzeno S, Cardozo C, Albertus J, Robins DM, Caplan AJ (2001) Functional interaction of human Cdc37 with the androgen receptor but not with the glucocorticoid receptor. J Biol Chem 276(8):5814–5820
Rauch JN, Gestwicki JE (2014) Binding of human nucleotide exchange factors to heat shock protein 70 (Hsp70) generates functionally distinct complexes in vitro. J Biol Chem 289(3):1402–1414
Rauch JN, Zuiderweg ER, Gestwicki JE (2016) Non-canonical interactions between heat shock cognate protein 70 (Hsc70) and Bcl2-associated anthanogene (BAG) co-chaperones are important for client release. J Biol Chem 291(38):19848–19857
Rauch JN, Tse E, Freilich R, Mok SA, Makley LN, Southworth DR, Gestwicki JE (2017) BAG3 is a modular, scaffolding protein that physically links heat shock protein 70 (Hsp70) to the small heat shock proteins. J Mol Biol 429(1):128–141
Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger JM, Garrido C, Kroemer G (2001) Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol 3:839–843
Raviol H, Sadlish H, Rodriguez F, Mayer MP, Bukau B (2006) Chaperone network in the yeast cytosol: Hsp110 is revealed as an Hsp70 nucleotide exchange factor. EMBO J 25(11):2510–2518
Ray S, Valekunja UK, Stangherlin A, Howell SA, Snijders AP, Damodaran G, Reddy AB (2020) Circadian rhythms in the absence of the clock gene Bmal1. Science 367(6479):800–806
Renoir J, Buchou T, Baulieu E (1986) Involvement of a non-hormone-binding 90-kilodalton protein in the nontransformed 8S form of the rabbit uterus progesterone receptor. Biochemistry 25:6405–6413
Retzlaff M, Hagn F, Mitschke L, Hessling M, Gugel F, Kessler H, Richter K, Buchner J (2010) Asymmetric activation of the hsp90 dimer by its cochaperone aha1. Mol Cell 37(3):344–354
Rhee YM, Sorin EJ, Jayachandran G, Lindahl E, Pande VS (2004) Simulations of the role of water in the protein-folding mechanism. Proc Natl Acad Sci USA 101(17):6456–6461
Richter K, Haslbeck M, Buchner J (2010) The heat shock response: life on the verge of death. Mol Cell 40(2):253–266
Riggs DL, Cox MB, Cheung-Flynn J, Prapapanich V, Carrigan PE, Smith DF (2004) Functional specificity of co-chaperone interactions with Hsp90 client proteins. Crit Rev Biochem Mol Biol 39(5–6):279–295
Rigo MM, Borges TJ, Lang BJ, Murshid A, Nitika D, Wolfgeher SK, Calderwood AWT, Bonorino C (2020) Host expression system modulates recombinant Hsp70 activity through post-translational modifications. FEBS J 287:4902
Rizzolo K, Huen J, Kumar A, Phanse S, Vlasblom J, Kakihara Y, Zeineddine HA, Minic Z, Snider J, Wang W, Pons C, Seraphim TV, Boczek EE, Alberti S, Costanzo M, Myers CL, Stagljar I, Boone C, Babu M, Houry WA (2017) Features of the chaperone cellular network revealed through systematic interaction mapping. Cell Rep 20(11):2735–2748
Rodina A, Wang T, Yan P, Gomes ED, Dunphy MP, Pillarsetty N, Koren J, Gerecitano JF, Taldone T, Zong H, Caldas-Lopes E, Alpaugh M, Corben A, Riolo M, Beattie B, Pressl C, Peter RI, Xu C, Trondl R, Patel HJ, Shimizu F, Bolaender A, Yang C, Panchal P, Farooq MF, Kishinevsky S, Modi S, Lin O, Chu F, Patil S, Erdjument-Bromage H, Zanzonico P, Hudis C, Studer L, Roboz GJ, Cesarman E, Cerchietti L, Levine R, Melnick A, Larson SM, Lewis JS, Guzman ML, Chiosis G (2016) The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature 538(7625):397–401
Rodriguez CF, Llorca O (2020) RPAP3 C-terminal domain: a conserved domain for the assembly of R2TP Co-chaperone complexes. Cells 9(5):1139
Ross CA, Poirier MA (2005) Opinion: What is the role of protein aggregation in neurodegeneration? Nat Rev Mol Cell Biol 6(11):891–898
Rudiger S, Buchberger A, Bukau B (1997a) Interaction of Hsp70 chaperones with substrates. Nat Struct Biol 4(5):342–349
Rudiger S, Germeroth L, Schneider-Mergener J, Bukau B (1997b) Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J 16(7):1501–1507
Salomons FA, Menendez-Benito V, Bottcher C, McCray BA, Taylor JP, Dantuma NP (2009) Selective accumulation of aggregation-prone proteasome substrates in response to proteotoxic stress. Mol Cell Biol 29(7):1774–1785
Sanchez M, Housley PR, Pratt WB (1986) The molybdate-stabilized glucocorticoid binding complex of L-cells contains a 98–100 KDalton steroid binding phosphoprotein and a 90 KDalton nonsteroid-binding phosphoprotein that is part of the murine heat-shock complex. J Steroid Biochem 24(1):9–18
Sanchez Y, Taulien J, Borkovich KA, Lindquist S (1992) Hsp104 is required for tolerance to many forms of stress. EMBO J 11:2357–2364
Santoro N, Johansson N, Thiele DJ (1998) Heat shock element architecture is an important determinant in the temperature and transactivation domain requirements for heat shock transcription factor. Mol Cell Biol 18(11):6340–6352
Santoro MG, Amici C, Rossi A (2009) Role of heat shock proteins in viral infection. Prokaryotic Eukaryot Heat Shock Proteins Infect Dis 4:51–84
Sarbeng EB, Liu Q, Tian X, Yang J, Li H, Wong JL, Zhou L, Liu Q (2015) A functional DnaK dimer is essential for the efficient interaction with Hsp40 heat shock protein. J Biol Chem 290(14):8849–8862
Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl FU, Moarefi I (2000) Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell 101(2):199–210
Schlecht R, Erbse AH, Bukau B, Mayer MP (2011) Mechanics of Hsp70 chaperones enables differential interaction with client proteins. Nat Struct Mol Biol 18(3):345–351
Schopf FH, Biebl MM, Buchner J (2017) The HSP90 chaperone machinery. Nat Rev Mol Cell Biol 18(6):345–360
Schwartz H, Scroggins B, Zuehlke A, Kijima T, Beebe K, Mishra A, Neckers L, Prince T (2015) Combined HSP90 and kinase inhibitor therapy: insights from the cancer genome atlas. Cell Stress Chaperones 20(5):729–741
Sciandra JJ, Subjeck JR (1984) Heat shock proteins and protection of proliferation and translation in mammalian cells. Cancer Res 44(11):5188–5194
Seo J, Han SY, Seong D, Han HJ, Song J (2019) Multifaceted C-terminus of HSP70-interacting protein regulates tumorigenesis via protein quality control. Arch Pharm Res 42(1):63–75
Sergent O, Pereira M, Belhomme C, Chevanne M, Huc L, Lagadic-Gossmann D (2005) Role for membrane fluidity in ethanol-induced oxidative stress of primary rat hepatocytes. J Pharmacol Exp Ther 313(1):104–111
Shang F, Taylor A (2011) Ubiquitin-proteasome pathway and cellular responses to oxidative stress. Free Radic Biol Med 51(1):5–16
Sharma SK, De los Rios P, Christen P, Lustig A, Goloubinoff P (2010) The kinetic parameters and energy cost of the Hsp70 chaperone as a polypeptide unfoldase. Nat Chem Biol 6(12):914–920
Shiau AK, Harris SF, Southworth DR, Agard DA (2006) Structural analysis of E. coli hsp90 reveals dramatic nucleotide-dependent conformational rearrangements. Cell 127(2):329–340
Shim E, Kim J, Bang E, Heo J, Lee J, Kim EH, Lee J, Park W, Kim S, Kim HJ, Smithies O, Jang J, Jin D, Seo J (2002) Targeted disruption of hsp70.1 sensitizes to osmotic stress. EMBO Rep 3(9):857–861
Shomura Y, Dragovic Z, Chang HC, Tzvetkov N, Young JC, Brodsky JL, Guerriero V, Hartl FU, Bracher A (2005) Regulation of Hsp70 function by HspBP1: structural analysis reveals an alternate mechanism for Hsp70 nucleotide exchange. Mol Cell 17(3):367–379
Sierra-Rivera E, Voorhees GJ, Freeman ML (1993) Gamma irradiation increases hsp-70 in Chinese hamster ovary cells. Radiat Res 135(1):40–45
Siligardi G, Panaretou B, Meyer P, Singh S, Woolfson DN, Piper PW, Pearl LH, Prodromou C (2002) Regulation of Hsp90 ATPase activity by the co-chaperone Cdc37p/p50cdc37. J Biol Chem 277(23):20151–20159
Singh IS, Hasday JD (2013) Fever, hyperthermia and the heat shock response. Int J Hyperthermia 29(5):423–435
Smock RG, Gierasch LM (2009) Sending signals dynamically. Science 324:198–203
Somasundaram T, Bhat SP (2000) Canonical heat shock element in the alpha B-crystallin gene shows tissue-specific and developmentally controlled interactions with heat shock factor. J Biol Chem 275(22):17154–17159
Somogyvari M, Gecse E, Soti C (2018) DAF-21/Hsp90 is required for C. elegans longevity by ensuring DAF-16/FOXO isoform A function. Sci Rep 8(1):12048
Sossey-Alaoui K, Kitamura E, Head K, Cowell JK (2002) Characterization of FAM10A4, a member of the ST13 tumor suppressor gene family that maps to the 13q14.3 region associated with B-Cell leukemia, multiple myeloma, and prostate cancer. Genomics 80(1):5–7
Spitz DR, Dewey WC, Li GC (1987) Hydrogen peroxide or heat shock induces resistance to hydrogen peroxide in Chinese hamster fibroblasts. J Cell Physiol 131(3):364–373
Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD (2005) Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem 280(46):38729–38739
Stankiewicz M, Nikolay R, Rybin V, Mayer MP (2010) CHIP participates in protein triage decisions by preferentially ubiquitinating Hsp70-bound substrates. FEBS J 277(16):3353–3367
Stein KC, Kriel A, Frydman J (2019) Nascent polypeptide domain topology and elongation rate direct the cotranslational hierarchy of Hsp70 and TRiC/CCT. Mol Cell 75(6):1117-1130 e5
Steinemann M, Schlosser A, Jank T, Aktories K (2018) The chaperonin TRiC/CCT is essential for the action of bacterial glycosylating protein toxins like Clostridium difficile toxins A and B. Proc Natl Acad Sci USA 115(38):9580–9585
Subjeck JR, Sciandra JJ, Chao CF, Johnson RJ (1982b) Heat shock proteins and biological response to hyperthermia. Br J Cancer Suppl 5:127–131
Subjeck JR, Sciandra JJ, Johnson RJ (1982a) Heat shock proteins and thermotolerance; a comparison of induction kinetics. Br J Radiol 55(656):579–584
Swain JF, Dinler G, Sivendran R, Montgomery DL, Stotz M, Gierasch LM (2007) Hsp70 chaperone ligands control domain association via an allosteric mechanism mediated by the interdomain linker. Mol Cell 26(1):27–39
Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover KD, Karras GI, Lindquist S (2012) Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell 150(5):987–1001
Takakuwa JE, Nitika LEK, Truman AW (2019) Oligomerization of Hsp70: current perspectives on regulation and function. Front Mol Biosci 6:81
Takeuchi T, Suzuki M, Fujikake N, Popiel HA, Kikuchi H, Futaki S, Wada K, Nagai Y (2015) Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc Natl Acad Sci USA 112(19):E2497–E2506
Taldone T, Wang T, Rodina A, Pillarsetty NVK, Digwal CS, Sharma S, Yan P, Joshi S, Pagare PP, Bolaender A, Roboz GJ, Guzman ML, Chiosis G (2020) A Chemical biology approach to the chaperome in cancer-HSP90 and beyond. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a034116.
Tamas MJ, Sharma SK, Ibstedt S, Jacobson T, Christen P (2014) Heavy metals and metalloids as a cause for protein misfolding and aggregation. Biomolecules 4(1):252–267
Tamas MJ, Fauvet B, Christen P, Goloubinoff P (2018) Misfolding and aggregation of nascent proteins: a novel mode of toxic cadmium action in vivo. Curr Genet 64(1):177–181
Tang D, Khaleque A, Jones EL, Theriault JR, Li C, Wong WH, Stevenson MA, Calderwood SK (2005) Expression of heat shock proteins and heat shock protein messenger ribonucleic acid in human prostate carcinoma in vitro and in tumors in vivo. Cell Stress Chaperones 10(1):46–58
Tanioka T, Nakatani Y, Kobayashi T, Tsujimoto M, Oh-ishi S, Murakami M, Kudo I (2003) Regulation of cytosolic prostaglandin E2 synthase by 90-kDa heat shock protein. Biochem Biophys Res Commun 303(4):1018–1023
Theodorakis NG, Morimoto RI (1987) Posttranscriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol Cell Biol 7(12):4357–4368
Theriault JR, Adachi H, Calderwood SK (2006) Role of scavenger receptors in the binding and internalization of heat shock protein 70. J Immunol 177(12):8604–8611
Tidwell JL, Houenou LJ, Tytell M (2004) Administration of Hsp70 in vivo inhibits motor and sensory neuron degeneration. Cell Stress Chaperones 9(1):88–98
Toth ME, Vigh L, Santha M (2014) Alcohol stress, membranes, and chaperones. Cell Stress Chaperones 19(3):299–309
Tranter M, Helsley RN, Paulding WR, McGuinness M, Brokamp C, Haar L, Liu Y, Ren X, Jones WK (2011) Coordinated post-transcriptional regulation of Hsp70.3 gene expression by microRNA and alternative polyadenylation. J Biol Chem 286(34):29828–29837
Trcka F, Durech M, Vankova P, Chmelik J, Martinkova V, Hausner J, Kadek A, Marcoux J, Klumpler T, Vojtesek B, Muller P, Man P (2019) Human stress-inducible Hsp70 has a high propensity to form ATP-dependent antiparallel dimers that are differentially regulated by cochaperone binding. Mol Cell Proteomics 18(2):320–337
Triandafillou CG, Katanski CD, Dinner AR, Drummond DA (2020) Transient intracellular acidification regulates the core transcriptional heat shock response. Elife 9:e54880
Tsen F, Bhatia A, O’Brien K, Cheng CF, Chen M, Hay N, Stiles B, Woodley DT, Li W (2013) Extracellular heat shock protein 90 signals through subdomain II and the NPVY motif of LRP-1 receptor to Akt1 and Akt2: a circuit essential for promoting skin cell migration in vitro and wound healing in vivo. Mol Cell Biol 33(24):4947–4959
Tulapurkar ME, Asiegbu BE, Singh IS, Hasday JD (2009) Hyperthermia in the febrile range induces HSP72 expression proportional to exposure temperature but not to HSF-1 DNA-binding activity in human lung epithelial A549 cells. Cell Stress Chaperones 14(5):499–508
Ungelenk S, Moayed F, Ho CT, Grousl T, Scharf A, Mashaghi A, Tans S, Mayer MP, Mogk A, Bukau B (2016) Small heat shock proteins sequester misfolding proteins in near-native conformation for cellular protection and efficient refolding. Nat Commun 7:13673
Van Durme J, Maurer-Stroh S, Gallardo R, Wilkinson H, Rousseau F, Schymkowitz J (2009) Accurate prediction of DnaK-peptide binding via homology modelling and experimental data. PLoS Comput Biol 5(8):e1000475
Van Oosten-Hawle P, Bolon DN, LaPointe P (2017) The diverse roles of Hsp90 and where to find them. Nat Struct Mol Biol 24(1):1–4
van Oosten-Hawle P, Morimoto RI (2014) Transcellular chaperone signaling: an organismal strategy for integrated cell stress responses. J Exp Biol 217(Pt 1):129–136
Varela AE, England KA, Cavagnero S (2019) Kinetic trapping in protein folding. Protein Eng Des Sel 32(2):103–108
Verba KA, Wang RY, Arakawa A, Liu Y, Shirouzu M, Yokoyama S, Agard DA (2016) Atomic structure of Hsp90-Cdc37-Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science 352(6293):1542–1547
Voellmy R (1994) Transduction of the stress signal and mechanisms of transcriptional regulation of heat shock/stress protein gene expression in higher eukaryotes. Crit Rev Eukaryot Gene Expr 4(4):357–401
Voellmy R (2004) On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones 9(2):122–133
Volloch V, Gabai VL, Rits S, Force T, Sherman MY (2000) HSP72 can protect cells from heat-induced apoptosis by accelerating the inactivation of stress kinase JNK. Cell Stress Chaperones 5(2):139–147
Wang RE (2011) Targeting heat shock proteins 70/90 and proteasome for cancer therapy. Curr Med Chem 18(27):4250–4264
Wang X, Grammatikakis N, Siganou A, Calderwood SK (2003) Regulation of molecular chaperone gene transcription involves the serine phosphorylation, 14-3-3 epsilon binding, and cytoplasmic sequestration of heat shock factor 1. Mol Cell Biol 23(17):6013–6026
Wang X, Khaleque MA, Zhao MJ, Zhong R, Gaestel M, Calderwood SK (2006) Phosphorylation of HSF1 by MAPK-activated protein kinase 2 on serine 121, inhibits transcriptional activity and promotes HSP90 binding. J Biol Chem 281(2):782–791
Wang T, Rodina A, Dunphy MP, Corben A, Modi S, Guzman ML, Gewirth DT, Chiosis G (2019) Chaperome heterogeneity and its implications for cancer study and treatment. J Biol Chem 294(6):2162–2179
Westerheide SD, Anckar J, Stevens SM Jr, Sistonen L, Morimoto RI (2009) Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 323(5917):1063–1066
Willmund F, del Alamo M, Pechmann S, Chen T, Albanese V, Dammer EB, Peng J, Frydman J (2013) The cotranslational function of ribosome-associated Hsp70 in eukaryotic protein homeostasis. Cell 152(1–2):196–209
Wirth D, Christians E, Munaut C, Dessy C, Foidart JM, Gustin P (2002) Differential heat shock gene hsp70-1 response to toxicants revealed by in vivo study of lungs in transgenic mice. Cell Stress Chaperones 7(4):387–395
Wisniewski J, Orosz A, Allada R, Wu C (1996) The C-terminal region of Drosophila heat shock factor (HSF) contains a constitutively functional transactivation domain. Nucleic Acids Res 24(2):367–374
Woo SK, Lee SD, Na KY, Park WK, Kwon HM (2002) TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity. Mol Cell Biol 22(16):5753–5760
Wu C (1995) Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol 11:441–469
Wu S, Hong L, Wang Y, Yu J, Yang J, Yang J, Zhang H, Perrett S (2020a) Kinetics of the conformational cycle of Hsp70 reveals the importance of the dynamic and heterogeneous nature of Hsp70 for its function. Proc Natl Acad Sci USA 117:7814–7823
Wu S, Hong L, Wang Y, Yu J, Yang J, Yang J, Zhang H, Perrett S (2020b) Kinetics of the conformational cycle of Hsp70 reveals the importance of the dynamic and heterogeneous nature of Hsp70 for its function. Proc Natl Acad Sci USA 117(14):7814–7823
Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, Benjamin IJ (1999) HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J 18(21):5943–5952
Xu Q, Schlett G, Li C, Hu Y, Wick G (2000) Mechanical stress-induced heat shock protein 70 expression in vascular smooth muscle cells is regulated by rac and ras small g proteins but not mitogen-activated protein kinases. Circ Res 86:112–1128
Yam AY, Xia Y, Lin HT, Burlingame A, Gerstein M, Frydman J (2008) Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nat Struct Mol Biol 15(12):1255–1262
Yamagishi N, Ishihara K, Saito Y, Hatayama T (2006) Hsp105 family proteins suppress staurosporine-induced apoptosis by inhibiting the translocation of Bax to mitochondria in HeLa cells. Exp Cell Res 312(17):3215–3223
Yao H, Haddad GG (2004) Calcium and pH homeostasis in neurons during hypoxia and ischemia. Cell Calcium 36(3–4):247–255
Zaarur N, Gabai VL, Porco JA Jr, Calderwood S, Sherman MY (2006) Targeting heat shock response to sensitize cancer cells to proteasome and Hsp90 inhibitors. Cancer Res 66(3):1783–1791
Zgajnar NR, De Leo SA, Lotufo CM, Erlejman AG, Piwien-Pilipuk G, Galigniana MD (2019) Biological actions of the Hsp90-binding immunophilins FKBP51 and FKBP52. Biomolecules 9(2):52
Zhang Y, Calderwood SK (2011) Autophagy, protein aggregation and hyperthermia: a mini-review. Int J Hyperthermia 27(5):409–414
Zhang Y, Murshid A, Prince T, Calderwood SK (2011) Protein kinase A regulates molecular chaperone transcription and protein aggregation. PLoS ONE 6(12):e28950
Zheng X, Krakowiak J, Patel N, Beyzavi A, Ezike J, Khalil AS, Pincus D (2016) Dynamic control of Hsf1 during heat shock by a chaperone switch and phosphorylation. Elife 5:e18638
Zheng X, Beyzavi A, Krakowiak J, Patel N, Khalil AS, Pincus D (2018) Hsf1 phosphorylation generates cell-to-cell variation in Hsp90 levels and promotes phenotypic plasticity. Cell Rep 22(12):3099–3106
Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, Hendrickson WA (1996) Structural analysis of substrate binding by the molecular chaperone DnaK. Science 272(5268):1606–1614
Zou J, Salminen WF, Roberts SM, Voellmy R (1998) Correlation between glutathione oxidation and trimerization of heat shock factor 1, an early step in stress induction of the Hsp response. Cell Stress Chaperones 3(2):130–141
Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R (1998a) Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94(4):471–480
Zwanzig R, Szabo A, Bagchi B (1992) Levinthal’s paradox. PNAS 89:20–22
Acknowledgements
We thank the Department of Radiation Oncology, BIDMC, Harvard Medical School for support and encouragement. Figures 1, 2, 3, 4, and 6 were created with biorender.com.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lang, B.J., Guerrero, M.E., Prince, T.L. et al. The functions and regulation of heat shock proteins; key orchestrators of proteostasis and the heat shock response. Arch Toxicol 95, 1943–1970 (2021). https://doi.org/10.1007/s00204-021-03070-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-021-03070-8