Abstract
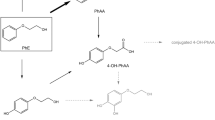
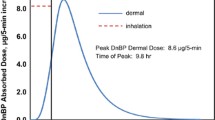
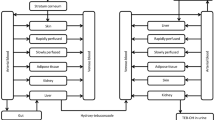
2-Phenoxyethanol (PE), ethylene glycol monophenyl ether, is widely used as a preservative in cosmetic products as well as in non-cosmetics. Since PE has been used in many types of products, it can be absorbed via dermal or inhaled route for systemic exposures. In this study, the pharmacokinetic (PK) studies of PE and its major metabolite, phenoxyacetic acid (PAA), after dermal (30 mg and 100 mg) and inhaled administration (77 mg) of PE in rats were performed. PE was administered daily for 4 days and blood samples were collected at day 1 and day 4 for PK analysis. PE was rapidly absorbed and extensively metabolized to form PAA. After multiple dosing, the exposures of PE and PAA were decreased presumably due to the induction of metabolizing enzymes of PE and PAA. In dermal mass balance study using [14C]-phenoxyethanol ([14C]PE) as a microtracer, most of the PE and its derivatives were excreted in urine (73.03%) and rarely found in feces (0.66%). Based on these PK results, a whole-body physiologically-based pharmacokinetic (PBPK) model of PE and PAA after dermal application and inhalation in rats was successfully developed. Most of parameters were obtained from the literatures and experiments, and intrinsic clearance at steady-state (CLint,ss) were optimized based on the observed multiple PK data. With the developed model, systemic exposures of PE and PAA after dermal application and inhalation were simulated following no-observed-adverse-effect level (NOAEL) of 500 mg/kg/day for dermal application and that of 12.7 mg/kg/day for inhalation provided by the Environmental Protection Agency. The area under the concentration–time curve at steady state (AUCss) in kidney and liver (and lung for inhalations), which are known target organs of exhibiting toxicity of PE, as well as AUCss in plasma of PE and PAA were obtained from the model.








Similar content being viewed by others
Data availability
The data are available upon the reasonable request to the corresponding author.
Abbreviations
- AC:
-
Aerosol concentration
- AMS:
-
Accelerator mass spectrometer
- AUC:
-
Area under the plasma concentration–time curve
- AUCinf :
-
Area under the plasma concentration–time curve from time zero to infinity
- AUClast :
-
Area under the plasma concentration–time curve within time span zero to last
- AUCPAA/AUCPE :
-
Metabolic ratio calculated by phenoxyacetic acid AUC/phenoxyethanol AUC ratio
- Carterial :
-
Drug concentrations in arterial blood compartments
- CL:
-
Clearance
- CLint :
-
Intrinsic clearance
- Cmax :
-
Maximum observed drug concentration in plasma
- Css :
-
Concentration at steady-state
- CT :
-
Concentration of drug in tissue
- CV:
-
Coefficient of variation
- CYP:
-
Cytochrome P450s
- D:
-
Duration of exposure to the aerosol
- ESI:
-
Electrospray ionization
- fu :
-
Unbound fraction in plasma
- fu ,hep :
-
Unbound fraction in hepatocytes
- IS:
-
Internal standard
- IVIVE:
-
In vitro-in vivo extrapolation
- Kper :
-
Permeability coefficient through skin
- Kp ,T :
-
Tissue:plasma partition coefficient
- LC–MS/MS:
-
Liquid chromatography tandem mass spectrometry
- LLOQ:
-
Lower limit of quantification
- LSC:
-
Liquid scintillation counting
- MRM:
-
Multiple reaction monitoring
- NOAEL:
-
No-observed-adverse-effect level
- p :
-
Original model parameter value
- PBPK:
-
Physiologically-based pharmacokinetic
- PK:
-
Pharmacokinetic
- QC:
-
Quality control
- QT :
-
Tissue blood flow
- RMV:
-
Represents respiratory minute volume, which is the respiratory rate multiplied by the tidal volume
- RSD:
-
Relative standard deviation
- SA:
-
Surface area of applied PE formulation
- t ½ :
-
Terminal elimination half-life
- Tmax :
-
Time to reach maximum (peak) plasma concentration following drug administration
- Vd :
-
Volume of distribution
- VT :
-
Volume in tissue compartment
References
Breslin WJ, Phillips JE, Lomax LG et al (1991) Hemolytic activity of ethylene glycol phenyl ether (EGPE) in rabbits. Fundam Appl Toxicol 17(3):466–481. https://doi.org/10.1016/0272-0590(91)90198-d
Chiou WL (1978) Critical evaluation of the potential error in pharmacokinetic studies of using the linear trapezoidal rule method for the calculation of the area under the plasma level–time curve. J Pharmacokinet Biopharm 6(6):539–546. https://doi.org/10.1007/BF01062108
Dreno B, Zuberbier T, Gelmetti C, Gontijo G, Marinovich M (2019) Safety review of phenoxyethanol when used as a preservative in cosmetics. J Eur Acad Dermatol Venereol 33(Suppl 7):15–24. https://doi.org/10.1111/jdv.15944
ECHA (2019) European Chemicals Agency (ECHA). Substance Infocard: 2-phenoxyethanol. https://echa.europa.eu/substance-information/-/substanceinfo/100.004.173
Elmokadem A, Riggs MM, Baron KT (2019) Quantitative systems pharmacology and physiologically-based pharmacokinetic modeling with mrgsolve: a hands-on tutorial. CPT Pharmacometrics Syst Pharmacol 8(12):883–893. https://doi.org/10.1002/psp4.12467
EPA (2019) Environmental Protection Agency (EPA). 2-phenoxyethanol; exemption from the requirement of a tolerance. Federal Register https://www.federalregister.gov/documents/2019/09/27/2019-20529/2-phenoxyethanol-exemption-from-the-requirement-of-a-tolerance
Feierman DE, Melinkov Z, Nanji AA (2003) Induction of CYP3A by ethanol in multiple in vitro and in vivo models. Alcohol Clin Exp Res 27(6):981–988. https://doi.org/10.1097/01.ALC.0000071738.53337.F4
Fioni A, Selg E, Cenacchi V et al (2018) Investigation of lung pharmacokinetic of the Novel PDE4 inhibitor CHF6001 in preclinical models: evaluation of the preciseinhale technology. J Aerosol Med Pulm Drug Deliv 31(1):61–70. https://doi.org/10.1089/jamp.2017.1369
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd edn. Marcel-Dekker, New York
Hanke N, Frechen S, Moj D et al (2018) PBPK models for CYP3A4 and P-gp DDI prediction: a modeling network of rifampicin, itraconazole, clarithromycin, midazolam, alfentanil, and digoxin. CPT Pharmacometrics Syst Pharmacol 7(10):647–659. https://doi.org/10.1002/psp4.12343
Howes D (1991) Absoprtion and metabolism of 2-phenoxyethanol in rat and man. 15th IFSCC Int Congr Cosmet Sci 3:415–434
Jones H, Rowland-Yeo K (2013) Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. CPT Pharmacometrics Syst Pharmacol 2:e63. https://doi.org/10.1038/psp.2013.41
Kim JM, Yoon JN, Jung JW et al (2013) Pharmacokinetics of hederacoside C, an active ingredient in AG NPP709, in rats. Xenobiotica 43(11):985–992. https://doi.org/10.3109/00498254.2013.788231
Kim TH, Kim MG, Kim MG et al (2015) Simultaneous determination of phenoxyethanol and its major metabolite, phenoxyacetic acid, in rat biological matrices by LC-MS/MS with polarity switching: application to ADME studies. Talanta 144:29–38. https://doi.org/10.1016/j.talanta.2015.05.075
Law FCP, Yao M, Bi HC, Lam S (2017) Physiologically based pharmacokinetic modeling of tea catechin mixture in rats and humans. Pharmacol Res Perspect 5(3):e00305. https://doi.org/10.1002/prp2.305
Lockley DJ, Howes D, Williams FM (2005) Cutaneous metabolism of glycol ethers. Arch Toxicol 79(3):160–168. https://doi.org/10.1007/s00204-004-0619-3
McLanahan ED, El-Masri HA, Sweeney LM, Kopylev LY, Clewell HJ, Wambaugh JF, Schlosser PM (2012) Physiologically based pharmacokinetic model use in risk assessment—why being published is not enough. Toxicol Sci 126(1):5–15
Ognibene TJ, Bench G, Vogel JS, Peaslee GF, Murov S (2003) A high-throughput method for the conversion of CO2 obtained from biochemical samples to graphite in septa-sealed vials for quantification of 14C via accelerator mass spectrometry. Anal Chem 75(9):2192–2196. https://doi.org/10.1021/ac026334j
Pawaskar DK, Straubinger RM, Fetterly GJ et al (2013) Physiologically based pharmacokinetic models for everolimus and sorafenib in mice. Cancer Chemother Pharmacol 71(5):1219–1229. https://doi.org/10.1007/s00280-013-2116-y
Rodgers T, Rowland M (2006) Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci 95(6):1238–1257. https://doi.org/10.1002/jps.20502
Roper CSHD, Blain PG, Williams FM (1997) Percutaneous penetration of 2-phenoxyethanol through rat and human skin. Food Chem Toxicol 35:1009–1016
SCCS (2016) Opinion on phenoxyethanol. Scientific Committee on Consumer Safety https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_195.pdf
Scognamiglio J, Jones L, Letizia CS, Api AM (2012) Fragrance material review on 2-phenoxyethanol. Food Chem Toxicol 50(Suppl 2):S244–S255. https://doi.org/10.1016/j.fct.2011.10.030
Scortichini BH, Quast JF, Rao KS (1987) Teratologic evaluation of 2-phenoxyethanol in New Zealand White rabbits following dermal exposure. Fundam Appl Toxicol 8(2):272–279. https://doi.org/10.1016/0272-0590(87)90126-6
Smith R, Jones RD, Ballard PG, Griffiths HH (2008) Determination of microsome and hepatocyte scaling factors for in vitro/in vivo extrapolation in the rat and dog. Xenobiotica 38(11):1386–1398. https://doi.org/10.1080/00498250802491662
Starek A, Szabla J, Kiec-Kononowicz K, Szymczak W (2008) Comparison of the in vitro hemolytic effects produced by alkoxyacetic acids on human and rat erythrocytes. Int J Occup Med Environ Health 21(2):147–155. https://doi.org/10.2478/v10001-008-0009-9
Starek-Swiechowicz B, Miranowicz-Dzierzawska K, Szymczak W, Budziszewska B, Starek A (2012) Hematological effects of exposure to mixtures of selected ethylene glycol alkyl ethers in rats. Pharmacol Rep 64(1):166–178. https://doi.org/10.1016/s1734-1140(12)70743-0
Thompson MD, Beard DA (2011) Development of appropriate equations for physiologically based pharmacokinetic modeling of permeability-limited and flow-limited transport. J Pharmacokinet Pharmacodyn 38(4):405–421. https://doi.org/10.1007/s10928-011-9200-x
Troutman JA, Rick DL, Stuard SB, Fisher J, Bartels MJ (2015) Development of a physiologically-based pharmacokinetic model of 2-phenoxyethanol and its metabolite phenoxyacetic acid in rats and humans to address toxicokinetic uncertainty in risk assessment. Regul Toxicol Pharmacol 73(2):530–543. https://doi.org/10.1016/j.yrtph.2015.07.012
Vogel JS, Giacomo JA, Schulze-Konig T, Keck BD, Lohstroh P, Dueker S (2010) Accelerator mass spectrometry best practices for accuracy and precision in bioanalytical (14)C measurements. Bioanalysis 2(3):455–468. https://doi.org/10.4155/bio.10.13
Winiwarter S, Chang G, Desai P et al (2019) Prediction of fraction unbound in microsomal and hepatocyte incubations: a comparison of methods across industry datasets. Mol Pharm 16(9):4077–4085. https://doi.org/10.1021/acs.molpharmaceut.9b00525
Wong BA (2007) Inhalation exposure systems: design, methods and operation. Toxicol Pathol 35(1):3–14. https://doi.org/10.1080/01926230601060017
Acknowledgements
We especially thank the support from Professor Kyung-Min Lim in Ewha Womans University, Professor Ok-Nam Bae in Hanyang University, and Professor Soo Kyung Bae in the Catholic University of Korea, Republic of Korea.
Funding
This study was funded by the Korea Ministry of Environment (MOE) as “the Environmental Health Action Program” and “Technology Program for establishing biocide safety management” (2019002490005 1485016231 and 2019002490004 1485016253) and also supported by a grant of the Korea Institute of Radiological and Medical Sciences (KIRAMS), funded by Ministry of Science and ICT(MSIT), Republic of Korea (No. 50539-2020).
Author information
Authors and Affiliations
Contributions
All authors were involved in the conception and design of the study and data interpretation. MK and SHB drafted the paper, conducted PK studies, and performed PBPK modeling. JBP and MK conducted PK studies and JS and CSY conducted sample analysis. All authors critically revised the paper and approved it for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to be disclosed.
Ethics approval
The animal experiment was approved by the Institutional Animal Care and Use Committee (IACUC) of the Korea Institute of Radiological & Medical Sciences (KIRAMS), Republic of Korea (approval number: Kirams2019-45). In addition, all animal experimental procedures were carried out in accordance with the revised Guidelines for Ethical Conduct in the care and Use of Animals and the rules of Good Laboratory Practice.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kwon, M., Park, J.B., Kwon, M. et al. Pharmacokinetics of 2-phenoxyethanol and its major metabolite, phenoxyacetic acid, after dermal and inhaled routes of exposure: application to development PBPK model in rats. Arch Toxicol 95, 2019–2036 (2021). https://doi.org/10.1007/s00204-021-03041-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-021-03041-z