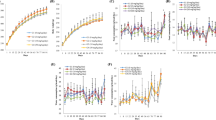
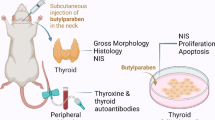
Abstract
Parabens are widely used preservatives in cosmetics and pharmaceutical products and are approved as food additives. These chemicals have been considered safe for many years. However, the literature classifies parabens as endocrine-disrupting chemicals, and an assessment of their influence on the endocrine system and systemic toxicity is important. This study explored long-term systemic toxicity, effects on the endocrine system, and toxicokinetic behavior after repeated subcutaneous administration of butylparaben to Sprague–Dawley rats. Rats were treated with vehicle (4% Tween 80) or butylparaben at dose levels of 2, 10, and 50 mg/kg/day for 13 weeks. Assessment of systemic toxicity and endocrine-disrupting effects was based on mortality; clinical signs; body weight; food and water consumption; ophthalmological findings; urinalysis; hematology and clinical biochemistry; organ weights; necropsy and histopathological findings; regularity and length of the estrous cycle; semen quality; and toxicokinetic behavior. Female uterine weight and estrous cycle, and male semen quality indicated no estrogenic effects. Butylparaben induced local irritation at the injection site in both sexes at a dose of 50 mg/kg/day, but systemic toxicity was not observed. Therefore, the no-observed-adverse-effect level of butylparaben is set at 50 mg/kg/day in rats of both sexes. Butylparaben was without endocrine system effects at this dose. Butylparaben displays dose-dependent systemic exposure up to the maximum dose of 50 mg/kg/day and repeated administration of butylparaben for 13 weeks shows no bioaccumulation.



Similar content being viewed by others
Change history
24 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00204-021-03054-8
References
Aarflot RL (2013) Human exposures to parabens in cosmetics—a literature study. Faculty of Health Sciences/Department of Community Medicine
Ana F, Paula FA (2016) parabens paradoxes in cosmetic formulations: a review. Int J Pharm Sci Res 6:1–21
Anderson JM, Rodriguez A, Chang DT (2008) Foreign body reaction to biomaterials. Semin Immunol 20:86–100
Aubert N (2009) Blood plasma pharmacokinetics and mass balance of yotal radioactivity in Sprague-Dawley rats following single administration of three different parabens (methyl-, butyl-, propyl-) by three different routes of administration (oral, dermal, sub-cutaneous). CIT, Centre International de Toxicologie, Evreux, France. Study No. 34851 PAR 26
Aubert N, Ameller T, Legrand JJ (2012) Systemic exposure to parabens: pharmacokinetics, tissue distribution, excretion balance and plasma metabolites of [14C]-methyl-, propyl- and butylparaben in rats after oral, topical or subcutaneous administration. Food Chem Toxicol 50:445–454
Boberg J, Taxvig C, Christiansen S et al (2010) Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol 30:301–312
Boberg J, Axelstad M, Svingen T et al (2016) Multiple endocrine disrupting effects in rats perinatally exposed to butylparaben. Toxicol Sci 152:244–256
Cosmetic Ingredient Review (2008) Report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol 27(Suppl 4):1–82
Darbre PD, Harvey PW (2008) Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J Appl Toxicol 28:561–578
Daston GP (2004) Developmental toxicity evaluation of butylparaben in Sprague-Dawley rats. Birth Defects Res B Dev Reprod Toxicol 71:296–302
Decker AH (2015) Associations between butylparaben and thyroid levels in females aged 12 and over (NHANES, 2007–2008). Georgia State University
Elder RL (1984) Final report on the safety assessment of methylparaben, ethylparaben, propylparaben, and butylparaben. J Am Coll Toxicol 3:147–209
Engeli RT, Rohrer SR, Vuorinen A et al (2007) Interference of paraben compounds with estrogen metabolism by inhibition of 17β-hydroxysteroid dehydrogenases. Int J Mol Sci 18:2007
Frederiksen H, Taxvig C, Hass U et al (2008) Higher levels of ethyl paraben and butyl paraben in rat amniotic fluid than in maternal plasma after subcutaneous administration. Toxicol Sci 106:376–383
Garcia T, Schreiber E, Kumar V et al (2017) Effects on the reproductive system of young male rats of subcutaneous exposure to n-butylparaben. Food Chem Toxicol 106:45–57
Golden R, Gandy J, Vollmer G (2005) A review of the endocrine activity of parabens and implications for potential risks to human health. Crit Rev Toxicol 35:435–458
Goswami P, Kalita JC (2013) Endocrine disrupting effects of butylparaben: a review. Int Res J Pharm 4:13–14
Guerra MT, Sanabria M, Leite GAA et al (2016) Maternal exposure to butyl paraben impairs testicular structure and sperm quality on male rats. Environ Toxicol 32:1273–1289
Han ZZ, Xu HD, Kim KH et al (2010) Reference data of the main physiological parameters in control sprague-dawley rats from pre-clinical toxicity studies. Lab Anim Res 26:153–164
Harrison PTC (2001) Endocrine disrupters and human health: current research will establish baseline indices. BMJ 323:1317–1318
Harvey PW (2005) Comment on developmental toxicity evaluation of butylparaben in Sprague-Dawley rats. Birth Defects Res B Dev Reprod Toxicol 74:114–115
Health Canada (2019) Changed to cosmetic ingredient hotlist. Accessed at https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restrictedingredients/changes.html#month20xx
Hoberman AM, Schreur DK, Leazer T et al (2008) Lack of effect of butylparaben and methylparaben on the reproductive system in male rats. Birth Defects Res B Dev Reprod Toxicol 83:123–133
Jones PS, Thigpen D, Morrison JL et al (1956) p-Hydroxybenzoic acid esters as preservatives III The physiological disposition of p-hydroxybenzoic acid and its esters. J Am Pharm Assoc Am Pharm Assoc 45:265–273
Kang KS, Che JH, Ryu DY et al (2002) Decreased sperm number and motile activity on the F1 offspring maternally exposed to butyl p-hydroxybenzoic acid (butyl paraben). J Vet Med Sci 64:227–235
Katzin WE, Centeno JA, Feng LJ et al (2005) Pathology of lymph nodes from patients with breast implants: a histologic and spectroscopic evaluation. Am J Surg Pathol 29:506–511
Kolatorova L, Duskova M, Vitku J et al (2017) Prenatal exposure to bisphenols and parabens and impacts on human physiology. Physiol Res 66(Suppl. 3):S305–S315
Kratofil RM, Kubes P, Deniset JF (2017) Monocyte conversion during inflammation and injury. Arterioscler Thromb Vasc Biol 37:35–42
Masten SA (2005) Butylparaben: Review of toxicological literature. National Institute of Environmental Health Sciences, North Carolina. https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/butylparaben_508.pdf
MFDS (The Ministry of Food and Drug Safety) (2013) The regulation on safety standards and others of cosmetics. MFDS Notification No. 2013-2 (in Korean) Accessed at https://www.mfds.go.kr/brd/m_207/view.do?seq=6139&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=107
MFDS (The Ministry of Food and Drug Safety) (2015) Toxicity test standards for medicines. MFDS Notification No. 2015-82 (in Korean) Accessed at https://mfds.go.kr/brd/m_211/view.do?seq=10128&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=51
Nohynek GJ, Borgert CJ, Dietrich D et al (2013) Endocrine disruption: Fact or urban legend? Toxicol Lett 22:295–305
OECD (1998) Repeated dose 90-day oral toxicity study in rodents. Guidelines for testing of chemicals. Test No. 408 Accessed at https://www.oecd-ilibrary.org/environment/test-no-408-repeated-dose-90-day-oral-toxicity-study-in-rodents_9789264070707-en
Oishi S (2001) Effects of butylparaben on the male reproductive system in rats. Toxicol Ind Health 17:31–39
Roberts GK, Waidyanatha S, Kisslinga GE et al (2016) Exposure to butyl paraben during gestation and lactation in Hsd: Sprague–dawley SD rats via dosed feed. Toxicol Rep 3:774–783
Routledge EJ, Parker J, Odum J et al (1998) Some alkyl hydroxy benzoate preservatives (Parabens) are estrogenic. Toxicol Appl Pharmacol 153:12–19
SCCS (Scientific Committee on Consumer Safety) (2010) Opinion on parabens. SCCS/1348/10. https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_041.pdf
SCCS (Scientific Committee on Consumer Safety) (2013) Opinion on parabens, updated request on propyl- and butylparaben. SCCS/1514/13. https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_132.pdf
Shaw J, deCatanzaro D (2009) Estrogenicity of parabens revisited: impact of parabens on early pregnancy and an uterotrophic assay in mice. Reprod Toxicol 28:26–31
Soni MG, Burdock GA, Taylor SL et al (2001) Safety assessment of propyl paraben: a review of the published literature. Food Chem Toxicol 39:513–532
Soni MG, Carabin IG, Burdock GA (2005) Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem Toxicol 43:985–1015
Taxvig C, Vinggaard AM, Hass U et al (2008) Do parabens have the ability to interfere with steroidogenesis? Toxicol Sci 106:206–213
U.S.FDA (U.S. Food and Drug Administration) (2020) Selected cosmetic ingredients prohibited & restricted Ingredients by FDA Regulations Accessed at https://www.fda.gov/cosmetics/cosmetics-laws-regulations/prohibited-restricted-ingredients-cosmetics
Yang J, Zhang L, Yu C et al (2014) Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res 2:1
Acknowledgements
This work was supported by Grants (14172MFDS975 and 19172MFDS221) from the Ministry of Food and Drug Safety, Korea in 2014 and 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bae, JS., Lee, J.D., Song, SW. et al. Thirteen-week subcutaneous repeated dose toxicity study of butylparaben and its toxicokinetics in rats. Arch Toxicol 95, 2037–2050 (2021). https://doi.org/10.1007/s00204-021-03037-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-021-03037-9