Abstract
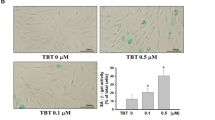
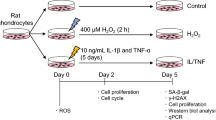
Arsenic-contaminated drinking water is known to be a serious human health problem. A previous epidemiological study has indicated that arsenic levels in blood were higher in arthritis patients compared to age-matched control subjects. Bone is known as an important arsenic store compartment in the body. Arsenic exposure has been suggested to promote senescence in human mesenchymal stem cells that may affect the balance of adipogenic and osteogenic differentiation. The toxicological effect and mechanism of arsenic exposure on articular chondrocytes still remain unclear. Here, we investigated the arsenic-induced senescence in cultured human articular chondrocytes and long-term arsenic-exposed rat articular cartilage. Arsenic trioxide (As2O3; 1–5 μM) significantly induced senescence in human articular chondrocytes by increasing senescence-associated β-galactosidase (SA-β-Gal) activity and protein expression of p16, p53, and p21. Arsenic induced the phosphorylation of p38 and c-Jun N-terminal kinase (JNK) proteins. The inhibitors of p38 and JNK significantly reversed the arsenic-induced chondrocyte senescence. Arsenic could also trigger the induction of GATA4-NF-κB signaling and senescence-associated secretory phenotype (SASP) by increasing IL-1α, IL-1β, TGF-β, TNF-α, CCL2, PAI-1, and MMP13 mRNA expression. The increased cartilage senescence and abrasion were also observed in a rat model long-term treatment with arsenic (0.05 and 0.5 ppm) in drinking water for 36 weeks as compared to age-matched control rats. The phosphorylation of p38 and JNK and the induction of GATA4-NF-κB signaling and SASP were enhanced in the rat cartilages. Taken together, these findings suggest that arsenic exposure is capable of inducing chondrocyte senescence and accelerating rat articular cartilage aging and abrasion.






Similar content being viewed by others
References
Afridi HI, Kazi TG, Kazi N, Talpur FN, Shah F et al (2013) Evaluation of status of arsenic, cadmium, lead and zinc levels in biological samples of normal and arthritis patients of age groups (46–60) and (61–75) years. Clin Lab 59:143–153
Akbal A, Yilmaz H, Tutkun E (2014) Arsenic exposure associated with decreased bone mineralization in male. Aging Male 17:256–258
Ashraf S, Cha BH, Kim JS, Ahn J, Han I, Park H et al (2016) Regulation of senescence associated signaling mechanisms in chondrocytes for cartilage tissue regeneration. Osteoarthritis Cartilage 24:196–205
Cao X, Luo P, Huang J, Liang C, He J, Wang Z et al (2019) Intraarticular senescent chondrocytes impair the cartilage regeneration capacity of mesenchymal stem cells. Stem Cell Res Ther 10:86
Centeno JA, Tseng CH, Van der Voet GB, Finkelman RB (2007) Global impacts of geogenic arsenic: a medical geology research case. Ambio 36:78–81
Chatterjee D, Bhattacharjee P, Sau TJ, Das JK, Sarma N, Bandyopadhyay AK et al (2015) Arsenic exposure through drinking water leads to senescence and alteration of telomere length in humans: A case-control study in west bengal, india. Mol Carcinog 54:800–809
Chen YJ, Chan DC, Lan KC, Wang CC, Chen CM, Chao SC et al (2015) Ppargamma is involved in the hyperglycemia-induced inflammatory responses and collagen degradation in human chondrocytes and diabetic mouse cartilages. J Orthop Res 33:373–381
Cheng H, Qiu L, Zhang H, Cheng M, Li W, Zhao X et al (2011) Arsenic trioxide promotes senescence and regulates the balance of adipogenic and osteogenic differentiation in human mesenchymal stem cells. Acta Biochim Biophys Sin (Shanghai) 43:204–209
Dani SU, Walter GF (2018) Chronic arsenic intoxication diagnostic score (casids). J Appl Toxicol 38:122–144
de Keizer PL, Packer LM, Szypowska AA, Riedl-Polderman PE, van den Broek NJ, de Bruin A et al (2010) Activation of forkhead box o transcription factors by oncogenic braf promotes p21cip1-dependent senescence. Cancer Res 70:8526–8536
Deshpande BR, Katz JN, Solomon DH, Yelin EH, Hunter DJ, Messier SP et al (2016) Number of persons with symptomatic knee osteoarthritis in the us: Impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res (Hoboken) 68:1743–1750
Dimozi A, Mavrogonatou E, Sklirou A, Kletsas D (2015) Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur Cell Mater 30:89–102 (discussion 103)
Fan Z, Soder S, Oehler S, Fundel K, Aigner T (2007) Activation of interleukin-1 signaling cascades in normal and osteoarthritic articular cartilage. Am J Pathol 171:938–946
Farr JN, Fraser DG, Wang H, Jaehn K, Ogrodnik MB, Weivoda MM et al (2016) Identification of senescent cells in the bone microenvironment. J Bone Miner Res 31:1920–1929
Gao SG, Zeng C, Li LJ, Luo W, Zhang FJ, Tian J et al (2016) Correlation between senescence-associated beta-galactosidase expression in articular cartilage and disease severity of patients with knee osteoarthritis. Int J Rheum Dis 19:226–232
He DS, Hu XJ, Yan YQ, Liu H (2017) Underlying mechanism of Sirt1 on apoptosis and extracellular matrix degradation of osteoarthritis chondrocytes. Mol Med Rep 16:845–850
Hong EH, Lee SJ, Kim JS, Lee KH, Um HD, Kim JH et al (2010) Ionizing radiation induces cellular senescence of articular chondrocytes via negative regulation of sirt1 by p38 kinase. J Biol Chem 285:1283–1295
Huang CF, Yang CY, Chan DC, Wang CC, Huang KH, Wu CC et al (2015) Arsenic exposure and glucose intolerance/insulin resistance in estrogen-deficient female mice. Environ Health Perspect 123:1138–1144
Huhmann BL, Harvey CF, Navas-Acien A, Graziano J, Parvez F, Chen Y et al (2019) Changes in arsenic exposure in araihazar, bangladesh from 2001 through 2015 following a blanket well testing and education campaign. Environ Int 125:82–89
Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP et al (2017) Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med 23:775–781
Jeon OH, David N, Campisi J, Elisseeff JH (2018) Senescent cells and osteoarthritis: a painful connection. J Clin Invest 128:1229–1237
Jun JI, Lau LF (2010) The matricellular protein ccn1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12:676–685
Kang C, Xu Q, Martin TD, Li MZ, Demaria M, Aron L et al (2015) The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 349:aaa5612
Kang SW, Kim J, Shin DY (2016) Inhibition of senescence and promotion of the proliferation of chondrocytes from articular cartilage by csa and fk506 involves inhibition of p38mapk. Mech Ageing Dev 153:7–13
Khairul I, Wang QQ, Jiang YH, Wang C, Naranmandura H (2017) Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget 8:23905–23926
Kim KH, Park B, Rhee DK, Pyo S (2015) Acrylamide induces senescence in macrophages through a process involving atf3, ros, p38/jnk, and a telomerase-independent pathway. Chem Res Toxicol 28:71–86
Kim HN, Chang J, Shao L, Han L, Iyer S, Manolagas SC et al (2017) DNA damage and senescence in osteoprogenitors expressing osx1 may cause their decrease with age. Aging Cell 16:693–703
Kuo HW, Kuo SM, Chou CH, Lee TC (2000) Determination of 14 elements in taiwanese bones. Sci Total Environ 255:45–54
Kuo CC, Weaver V, Fadrowski JJ, Lin YS, Guallar E, Navas-Acien A (2015) Arsenic exposure, hyperuricemia, and gout in us adults. Environ Int 76:32–40
Ma Y, Qi M, An Y, Zhang L, Yang R, Doro DH et al (2018) Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell 17:e12709
Mannan T, Ahmed S, Akhtar E, Ahsan KB, Haq A, Kippler M et al (2018) Associations of arsenic exposure with telomere length and naive t cells in childhood-a birth cohort study. Toxicol Sci 164:539–549
McHugh D, Gil J (2018) Senescence and aging: Causes, consequences, and therapeutic avenues. J Cell Biol 217:65–77
Medina-Pizzali M, Robles P, Mendoza M, Torres C (2018) [Arsenic intake: Impact in human nutrition and health]. Rev Peru Med Exp Salud Publica 35:93–102
Nielsen GP, Stemmer-Rachamimov AO, Shaw J, Roy JE, Koh J, Louis DN (1999) Immunohistochemical survey of p16INK4A expression in normal human adult and infant tissues. Lab Invest 79:1137–1143
Sacitharan PK (2019) Ageing and osteoarthritis. Subcell Biochem 91:123–159
Sharma S, Kaur I, Nagpal AK (2017) Assessment of arsenic content in soil, rice grains and groundwater and associated health risks in human population from ropar wetland, india, and its vicinity. Environ Sci Pollut Res Int 24:18836–18848
Sorrentino JA, Krishnamurthy J, Tilley S, Alb JG Jr, Burd CE, Sharpless NE (2014) P16ink4a reporter mice reveal age-promoting effects of environmental toxicants. J Clin Invest 124:169–173
Szymczyk KH, Kerr BA, Freeman TA, Adams CS, Steinbeck MJ (2006) Involvement of hydrogen peroxide in the differentiation and apoptosis of preosteoclastic cells exposed to arsenite. Biochem Pharmacol 72:761–769
Trachana V, Mourmoura E, Papathanasiou I, Tsezou A (2019) Understanding the role of chondrocytes in osteoarthritis: Utilizing proteomics. Expert Rev Proteomics 16:201–213
Tutkun L, Gunduzoz M, Turksoy VA, Deniz S, Oztan O, Cetintepe SP et al (2019) Arsenic-induced inflammation in workers. Mol Biol Rep 46:2371–2378
Vinatier C, Domínguez E, Guicheux J, Caramés B (2018) Role of the inflammation-autophagy-senescence integrative network in osteoarthritis. Front Physiol 9:706
Wang T, Yuan Y, Zou H, Yang J, Zhao S, Ma Y et al (2016) The ER stress regulator Bip mediates cadmium-induced autophagy and neuronal senescence. Sci Rep 6:38091
WHO Arsenichttps://www.who.int/news-room/fact-sheets/detail/arsenic Accessed Jun 20, 2019.
Wu CT, Lu TY, Chan DC, Tsai KS, Yang RS, Liu SH (2014) Effects of arsenic on osteoblast differentiation in vitro and on bone mineral density and microstructure in rats. Environ Health Perspect 122:559–565
Yamaguchi Y, Madhyastha H, Madhyastha R, Choijookhuu N, Hishikawa Y, Pengjam Y et al (2016) Arsenic acid inhibits proliferation of skin fibroblasts, and increases cellular senescence through ros mediated mst1-foxo signaling pathway. J Toxicol Sci 41:105–113
Yen YP, Tsai KS, Chen YW, Huang CF, Yang RS, Liu SH (2010) Arsenic inhibits myogenic differentiation and muscle regeneration. Environ Health Perspect 118:949–956
Yi JK, Kim HJ, Yu DH, Park SJ, Shin MJ, Yuh HS et al (2012) Regulation of inflammatory responses and fibroblast-like synoviocyte apoptosis by calcineurin-binding protein 1 in mice with collagen-induced arthritis. Arthritis Rheum 64:2191–2200
Zhang Y, Xu B, Guo Z, Han J, Li H, Jin L et al (2019) Human health risk assessment of groundwater arsenic contamination in jinghui irrigation district, china. J Environ Manage 237:163–169
Zindy F, Quelle DE, Roussel MF, Sherr CJ (1997) Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene 15:203–211
Acknowledgements
This study was funded by grants from Ministry of Science and Technology of Taiwan (NSC101-2314-B-002-118-MY2; MOST105-2314-B-002-009-MY3).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chung, YP., Chen, YW., Weng, TI. et al. Arsenic induces human chondrocyte senescence and accelerates rat articular cartilage aging. Arch Toxicol 94, 89–101 (2020). https://doi.org/10.1007/s00204-019-02607-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-019-02607-2