Abstract
The occurrence of idiosyncratic drug-induced liver injury (IDILI) is a leading cause of post-marketing safety warnings and withdrawals of drugs. Carbamazepine (CBZ), widely used as an antiepileptic agent, could cause rare but severe idiosyncratic liver injury in humans. Although recent studies have shown that inflammasome is implicated in CBZ-induced hepatocellular injury in vitro, the precise pathogenesis of hepatotoxicity remains largely unexplored. Here we report that CBZ causes idiosyncratic liver injury through promoting specific stimuli-induced NLRP3 inflammasome activation. CBZ (40 μM) enhances NLRP3 inflammasome activation triggered by adenosine triphosphate (ATP) or nigericin, rather than SiO2, monosodium urate crystal or intracellular lipopolysaccharide (LPS). In addition, CBZ has no effect on NLRC4 or AIM2 inflammasome activation. Mechanistically, synergistic induction of mitochondrial reactive oxygen species (mtROS) is a crucial event in the enhancement effect of CBZ on ATP- or nigericin-induced NLRP3 inflammasome activation. Moreover, the “C=C” on the seven-membered ring and “C=O” on the nitrogen of CBZ may be contribute to NLRP3 inflammasome hyperactivation and hepatotoxicity. Notably, in vivo data indicate that CBZ (50 mg/kg) causes liver injury in an LPS (2 mg/kg)-mediated susceptibility mouse model of IDILI, accompanied by an increase in caspase-1 activity and IL-1β production, whereas the combination of CBZ and LPS does not exhibit the effect in NLRP3-knockout mice. In conclusion, CBZ specifically promotes ATP- or nigericin-induced NLRP3 inflammasome activation and causes idiosyncratic liver injury. Our findings also suggest that CBZ may be avoided in patients with NLRP3 inflammasome activation-related diseases that are triggered by ATP or nigericin, which may be risk factors for IDILI.






Similar content being viewed by others
Abbreviations
- IDILI:
-
Idiosyncratic drug-induced liver injury
- ATP:
-
Adenosine triphosphate
- CBZ:
-
Carbamazepine
- MSU:
-
Monosodium urate crystal
- LPS:
-
Lipopolysaccharide
- mtROS:
-
Mitochondrial reactive oxygen species
- PAMPs:
-
Pathogen-associated molecular patterns
- DAMPs:
-
Damage-associated molecular patterns
- PMA:
-
Phorbol-12-myristate-13-acetate
- BMDMS :
-
Bone-marrow-derived macrophages
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate transaminase
- LDH:
-
Lactate dehydrogenase
- HBSS:
-
Hank’s balanced salt solution
References
Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H (2010) Mechanisms of immune-mediated liver injury. Toxicol Sci 115(2):307–321. https://doi.org/10.1093/toxsci/kfq009
Bjornsson ES (2015) Drug-induced liver injury: an overview over the most critical compounds. Arch Toxicol 89(3):327–334. https://doi.org/10.1007/s00204-015-1456-2
Bjornsson ES (2016) Hepatotoxicity by drugs: the most common implicated agents. Int J Mol Sci 17(2):224. https://doi.org/10.3390/ijms17020224
Buchweitz JP, Ganey PE, Bursian SJ, Roth RA (2002) Underlying endotoxemia augments toxic responses to chlorpromazine: is there a relationship to drug idiosyncrasy? J Pharmacol Exp Ther 300(2):460–467. https://doi.org/10.1124/jpet.300.2.460
Chalasani N, Bjornsson E (2010) Risk factors for idiosyncratic drug-induced liver injury. Gastroenterology 138(7):2246–2259. https://doi.org/10.1053/j.gastro.2010.04.001
Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ (2014) ACG Clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 109(7):950–966. https://doi.org/10.1038/ajg.2014.131(quiz 967)
Chalasani N, Bonkovsky HL, Fontana R et al (2015) Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology 148(7):1340-52.e7. https://doi.org/10.1053/j.gastro.2015.03.006
Chen YL, Xu G, Liang X et al (2016) Inhibition of hepatic cells pyroptosis attenuates CLP-induced acute liver injury. Am J Transl Res 8(12):5685–5695
Cho T, Uetrecht J (2017) How reactive metabolites induce an immune response that sometimes leads to an idiosyncratic drug reaction. Chem Res Toxicol 30(1):295–314. https://doi.org/10.1021/acs.chemrestox.6b00357
Deng X, Stachlewitz RF, Liguori MJ et al (2006) Modest inflammation enhances diclofenac hepatotoxicity in rats: role of neutrophils and bacterial translocation. J Pharmacol Exp Ther 319(3):1191–1199. https://doi.org/10.1124/jpet.106.110247
Fontana RJ (2014) Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology 146(4):914–928. https://doi.org/10.1053/j.gastro.2013.12.032
Ganey PE, Luyendyk JP, Maddox JF, Roth RA (2004) Adverse hepatic drug reactions: inflammatory episodes as consequence and contributor. Chem Biol Interact 150(1):35–51. https://doi.org/10.1016/j.cbi.2004.09.002
Garcia-Cortes M, Ortega-Alonso A, Lucena MI, Andrade RJ (2018) Drug-induced liver injury: a safety review. Expert Opin Drug Saf 17(8):795–804. https://doi.org/10.1080/14740338.2018.1505861
Geng Y, Ma Q, Liu YN et al (2015) Heatstroke induces liver injury via IL-1beta and HMGB1-induced pyroptosis. J Hepatol 63(3):622–633. https://doi.org/10.1016/j.jhep.2015.04.010
Gomez-Ibanez A, Serratosa JM, Guillamon E et al (2017) Efficacy and safety of eslicarbazepine-acetate in elderly patients with focal epilepsy: case series. Seizure 48:53–56. https://doi.org/10.1016/j.seizure.2017.04.003
Hammad MA, Abdel-Bakky MS, Walker LA, Ashfaq MK (2011) Oxidized low-density lipoprotein and tissue factor are involved in monocrotaline/lipopolysaccharide-induced hepatotoxicity. Arch Toxicol 85(9):1079–1089. https://doi.org/10.1007/s00204-011-0649-6
He Y, Hara H, Nunez G (2016) Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 41(12):1012–1021. https://doi.org/10.1016/j.tibs.2016.09.002
He H, Jiang H, Chen Y et al (2018) Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun 9(1):2550. https://doi.org/10.1038/s41467-018-04947-6
Holt MP, Ju C (2006) Mechanisms of drug-induced liver injury. AAPS J 8(1):E48–E54. https://doi.org/10.1208/aapsj080106
Honda M, Takeichi T, Asonuma K, Tanaka K, Kusunoki M, Inomata Y (2013) Intravital imaging of neutrophil recruitment in hepatic ischemia–reperfusion injury in mice. Transplantation 95(4):551–558. https://doi.org/10.1097/TP.0b013e31827d62b5
Huang Y, Jiang H, Chen Y et al (2018) Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol Med. https://doi.org/10.15252/emmm.201708689
Jaeschke H (2011) Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol 26(Suppl 1):173–179. https://doi.org/10.1111/j.1440-1746.2010.06592.x
Ju C, Reilly T (2012) Role of immune reactions in drug-induced liver injury (DILI). Drug Metab Rev 44(1):107–115. https://doi.org/10.3109/03602532.2011.645579
Kato R, Uetrecht J (2017) Supernatant from hepatocyte cultures with drugs that cause idiosyncratic liver injury activates macrophage inflammasomes. Chem Res Toxicol 30(6):1327–1332. https://doi.org/10.1021/acs.chemrestox.7b00065
Kato R, Ijiri Y, Hayashi T, Uetrecht J (2019) The 2-hydroxyiminostilbene metabolite of carbamazepine or the supernatant from incubation of hepatocytes with carbamazepine activates inflammasomes; implications for carbamazepine-induced hypersensitivity reactions. Drug Metab Dispos. https://doi.org/10.1124/dmd.119.087981
Kayagaki N, Warming S, Lamkanfi M et al (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479(7371):117–121. https://doi.org/10.1038/nature10558
Kubes P, Mehal WZ (2012) Sterile inflammation in the liver. Gastroenterology 143(5):1158–1172. https://doi.org/10.1053/j.gastro.2012.09.008
Lamkanfi M, Dixit VM (2014) Mechanisms and functions of inflammasomes. Cell 157(5):1013–1022. https://doi.org/10.1016/j.cell.2014.04.007
Lu J, Roth RA, Malle E, Ganey PE (2013) Roles of the hemostatic system and neutrophils in liver injury from co-exposure to amiodarone and lipopolysaccharide. Toxicol Sci 136(1):51–62. https://doi.org/10.1093/toxsci/kft170
Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L (2014) The role of oxidative stress during inflammatory processes. Biol Chem 395(2):203–230. https://doi.org/10.1515/hsz-2013-0241
Luyendyk JP, Maddox JF, Cosma GN, Ganey PE, Cockerell GL, Roth RA (2003) Ranitidine treatment during a modest inflammatory response precipitates idiosyncrasy-like liver injury in rats. J Pharmacol Exp Ther 307(1):9–16. https://doi.org/10.1124/jpet.103.054288
Luyendyk JP, Maddox JF, Green CD, Ganey PE, Roth RA (2004) Role of hepatic fibrin in idiosyncrasy-like liver injury from lipopolysaccharide-ranitidine coexposure in rats. Hepatology 40(6):1342–1351. https://doi.org/10.1002/hep.20492
Mak A, Uetrecht J (2015) The combination of anti-CTLA-4 and PD1−/− mice unmasks the potential of isoniazid and nevirapine to cause liver injury. Chem Res Toxicol 28(12):2287–2291. https://doi.org/10.1021/acs.chemrestox.5b00305
Metushi IG, Hayes MA, Uetrecht J (2015) Treatment of PD-1(−/−) mice with amodiaquine and anti-CTLA4 leads to liver injury similar to idiosyncratic liver injury in patients. Hepatology 61(4):1332–1342. https://doi.org/10.1002/hep.27549
Mills EL, Kelly B, O’Neill LAJ (2017) Mitochondria are the powerhouses of immunity. Nat Immunol 18(5):488–498. https://doi.org/10.1038/ni.3704
Minutoli L, Puzzolo D, Rinaldi M et al (2016) ROS-mediated NLRP3 inflammasome activation in brain, heart, kidney, and testis ischemia/reperfusion injury. Oxidative Med Cell Longev 2016:2183026. https://doi.org/10.1155/2016/2183026
Mridha AR, Wree A, Robertson AAB et al (2017) NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol 66(5):1037–1046. https://doi.org/10.1016/j.jhep.2017.01.022
Nicoletti P, Aithal GP, Bjornsson ES et al (2017) Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Gastroenterology 152(5):1078–1089. https://doi.org/10.1053/j.gastro.2016.12.016
Oliveira THC, Marques PE, Proost P, Teixeira MMM (2018) Neutrophils: a cornerstone of liver ischemia and reperfusion injury. Lab Investig 98(1):51–62. https://doi.org/10.1038/labinvest.2017.90
Petrasek J, Bala S, Csak T et al (2012) IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Investig 122(10):3476–3489. https://doi.org/10.1172/jci60777
Real M, Barnhill MS, Higley C, Rosenberg J, Lewis JH (2019) Drug-induced liver injury: highlights of the recent literature. Drug Saf 42(3):365–387. https://doi.org/10.1007/s40264-018-0743-2
Schwabe RF, Brenner DA (2006) Mechanisms of liver injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol 290(4):G583–G589. https://doi.org/10.1152/ajpgi.00422.2005
Shaw PJ, Ditewig AC, Waring JF et al (2009) Coexposure of mice to trovafloxacin and lipopolysaccharide, a model of idiosyncratic hepatotoxicity, results in a unique gene expression profile and interferon gamma-dependent liver injury. Toxicol Sci 107(1):270–280. https://doi.org/10.1093/toxsci/kfn205
Song N, Liu ZS, Xue W et al (2017) NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol Cell 68(1):185-197.e6. https://doi.org/10.1016/j.molcel.2017.08.017
Tangamornsuksan W, Chaiyakunapruk N, Somkrua R, Lohitnavy M, Tassaneeyakul W (2013) Relationship between the HLA-B*1502 allele and carbamazepine-induced Stevens–Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol 149(9):1025–1032. https://doi.org/10.1001/jamadermatol.2013.4114
Tilg H, Moschen AR, Szabo G (2016) Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 64(3):955–965. https://doi.org/10.1002/hep.28456
Wang Z, Xu G, Gao Y et al (2019) Cardamonin from a medicinal herb protects against LPS-induced septic shock by suppressing NLRP3 inflammasome. Acta Pharm Sin B 9(4):734–744. https://doi.org/10.1016/j.apsb.2019.02.003
Weston JK, Uetrecht J (2014) Activation of inflammasomes by agents causing idiosyncratic skin reactions: a possible biomarker. Chem Res Toxicol 27(6):949–951. https://doi.org/10.1021/tx5001333
Wree A, Eguchi A, McGeough MD et al (2014) NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 59(3):898–910. https://doi.org/10.1002/hep.26592
You Q, Cheng L, Reilly TP, Wegmann D, Ju C (2006) Role of neutrophils in a mouse model of halothane-induced liver injury. Hepatology 44(6):1421–1431. https://doi.org/10.1002/hep.21425
Zhang C, Wang G, Fu M (2018a) 934 Carbamazepine-10,11-epoxide drives CD8+ T-cell skin trafficking through NLRP3 inflammasome upregulation and contributes to necroptosis of keratinocytes in severe cutaneous adverse drug reactions. J Investig Dermatol 138(5, Supplement):S159. https://doi.org/10.1016/j.jid.2018.03.946
Zhang X, Luan J, Chen W et al (2018b) Mesoporous silica nanoparticles induced hepatotoxicity via NLRP3 inflammasome activation and caspase-1-dependent pyroptosis. Nanoscale 10(19):9141–9152. https://doi.org/10.1039/c8nr00554k
Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469(7329):221–225. https://doi.org/10.1038/nature09663
Acknowledgements
This work has been supported by grants from National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program” (2017ZX09301022, 2018ZX09101002-001-002), National Natural Science Foundation of China (81874368, 81630100, 81903891), Beijing Nova Program (Z181100006218001), Innovation Groups of the National Natural Science Foundation of China (81721002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
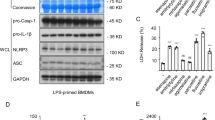
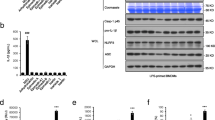
CBZ promotes the activation of NLRP3 inflammasome triggered by ATP, an effect that is not present in NLRP3-/- BMDMs. (a) BMDMs or LPS-primed BMDMs were treated with ATP, CBZ, or ATP plus CBZ. The secretion of TNF-α was detected in SN. (b) LPS-primed BMDMs were treated with various doses of CBZ and then stimulated with ATP. The secretion of TNF-α was detected in SN. (c-g) LPS-primed wild type (WT) BMDMs or NLRP3 knock out (NLRP3-/-) BMDMs were treated with ATP in the presence or absence of CBZ. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, ASC in WCL (c). Caspase-1 activity (d), secretion of IL-1β (e), LDH (f), TNF-α (g) in SN from WT BMDMs and NLRP3-/- BMDMs described in (c). Data are expressed as mean ± SEM (n=3) from three independent experiments with biological duplicates in (a, b, d-g). Statistics differences were analyzed using an unpaired Student’s t-test: **P < 0.01, ***P < 0.001 vs. the WT ATP group. Supplementary Fig. 2 CBZ has no influence on the cell viability in BMDMs and cultured supernatant ALT and AST in L02 cells. (a) BMDMs were incubated at 37°C followed by treatment with CBZ for 24 h, then these cells were cultured with CCK-8 for 30 min. The optical density values at the wavelength of 450 nm were determined. (b, c) L02 cells were seeded in 96-well growth-medium plate overnight at 1×105 cells/well. Next, the cells were incubated by CBZ treatment for 24 h, then the cultured supernatant ALT (b) and AST (c) were determined. APAP, acetaminophen. Statistics differences were analyzed using one-way ANOVE: ***P < 0.001 vs. the control group. Supplementary Fig. 3 CBZ promotes NLRP3 inflammasome activation stimulated by nigericin in BMDMs and THP-1 cells. (a, b) LPS-primed BMDMs were treated with various doses of CBZ and then stimulated with nigericin. The secretion of LDH (a), TNF-α (b) were detected in SN. (c) PMA-primed THP-1 cells were stimulated with nigericin after CBZ treatment. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, ASC in WCL. (d-g) Caspase-1 activity (d), secretion of IL-1β (e), LDH (f), TNF-α (g) in SN from THP-1 cells described in (c). Data are expressed as mean ± SEM (n=3) from three independent experiments with biological duplicates in (a, b, d-g). Statistics differences were analyzed using one-way ANOVA: *P < 0.05, **P < 0.01, ***P < 0.001 vs. the LPS or PMA plus nigericin group. Supplementary Fig. 4 CBZ has no effect on the activation of NLRP3 inflammasome induced by MSU, SiO2 and intracellular LPS, as well as AIM2 and NLRC4 inflammasome. (a) LPS-primed BMDMs were treated with various doses of CBZ and then stimulated with MSU or ATP. The secretion of TNF-α was detected in SN. (b) LPS-primed BMDMs were treated with CBZ and then stimulated with SiO2 or ATP. The secretion of TNF-α was detected in SN. (c) Pam3CSK4-primed BMDMs were treated with various doses of CBZ and then stimulated with LPS, or LPS-primed BMDMs were treated with CBZ and then stimulated with ATP. The secretion of TNF-α was detected in SN. (d) LPS-primed BMDMs were treated with CBZ and then stimulated with ATP, poly(dA:dT) or Lfn-Flic. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, ASC in WCL. (e-g) Caspase-1 activity (e), secretion of IL-1β (f), TNF-α (g) in SN described in (d). Data are expressed as mean ± SEM (n=3) from three independent experiments with biological duplicates in (a-c, f-h). Statistics differences were analyzed using an unpaired Student’s t-test: **P < 0.01, ***P < 0.001 vs. the ATP group. Supplementary Fig. 5 CBZ has no effect on intracellular potassium. (a, b) Qualification of potassium efflux in LPS-primed BMDMs treated with CBZ and then stimulated with different stimuli. Supplementary Fig. 6 CBZ facilitates ATP/nigericin-induced mitochondrial reactive oxygen species (mtROS) production. LPS-primed BMDMs were treated with CBZ before stimulated by ATP, nigericin or SiO2. For mtROS measurement, BMDMs were loaded with MitoSOX red mitochondrial superoxide indicator (Ex/Em: 510/580 nm). After staining and washing, flow cytometry was conducted to test mtROS. Supplementary Fig. 7 The effect of H2O2 on NLRP3 inflammasome activation triggered by ATP, nigericin, and SiO2. (a-c) LPS-primed BMDMs were treated with H2O2 and then stimulated with ATP (a), nigericin (b) or SiO2(c). Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN. Data are expressed as mean ± SEM (n=3) from three independent experiments with biological duplicates. Statistics differences were analyzed using one-way ANOVA: *P < 0.05, **P < 0.01, ***P < 0.001 vs. the ATP or nigericin group (PDF 1217 kb)
Rights and permissions
About this article
Cite this article
Wang, Z., Xu, G., Zhan, X. et al. Carbamazepine promotes specific stimuli-induced NLRP3 inflammasome activation and causes idiosyncratic liver injury in mice. Arch Toxicol 93, 3585–3599 (2019). https://doi.org/10.1007/s00204-019-02606-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-019-02606-3