Abstract
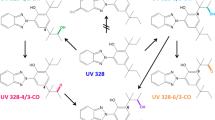
Octocrylene (OC) is a UV filter used in sun screens and other personal care products, but also in polymers and food contact materials for stabilization. In this study, we investigate human OC metabolism and urinary excretion after oral dosage of approx. 5 mg OC [≙ 61.8–89.5 µg/(kg body weight)] in three male volunteers. In a screening approach, we tentatively identified six urinary OC metabolites. For three, renal elimination kinetics was quantitatively investigated using authentic standards: the sidechain oxidation product 2-ethyl-5-hydroxyhexyl 2-cyano-3,3-diphenyl acrylate (5OH–OC), the beta-oxidation product 2-(carboxymethyl)butyl 2-cyano-3,3-diphenyl acrylate (dinor OC carboxylic acid; DOCCA), and the ester hydrolysis product 2-cyano-3,3-diphenylacrylic acid (CPAA). CPAA was the major urinary metabolite, representing 45% (range 40–50%) of the OC dose. 5OH–OC and DOCCA were only minor metabolites with low, but highly consistent renal conversion factors of 0.008% (0.005–0.011%) and 0.13% (0.11–0.16%), respectively. Peak urinary metabolite concentrations were observed between 3.2 h and 4.2 h postdose. All three metabolites were excreted with biphasic elimination kinetics, with considerably longer elimination half-lives for DOCCA (1st phase: 3.0 h; 2nd phase: 16 h) and CPAA (5.7 h and 16 h) compared to 5OH–OC (1.3 h and 6.4 h). 99% of all 5OH–OC was excreted within 24 h compared to 82% of DOCCA and 77% of CPAA. After dermal exposure, we detected the same metabolites with similar ratios in urine, however, at much lower concentrations and with considerably delayed elimination.




Similar content being viewed by others
References
Angerer J, Ewers U, Wilhelm M (2007) Human biomonitoring: state of the art. Int J Hyg Environ Health 210(3–4):201–228. https://doi.org/10.1016/j.ijheh.2007.01.024
Angerer J, Aylward LL, Hays SM, Heinzow B, Wilhelm M (2011) Human biomonitoring assessment values: approaches and data requirements. Int J Hyg Environ Health 214(5):348–360. https://doi.org/10.1016/j.ijheh.2011.06.002
Ao J, Yuan T, Ma Y, Gao L, Ni N, Li D (2017) Identification, characteristics and human exposure assessments of triclosan, bisphenol-A, and four commonly used organic UV filters in indoor dust collected from Shanghai, China. Chemosphere 184:575–583. https://doi.org/10.1016/j.chemosphere.2017.06.033
Apel P, Angerer J, Wilhelm M, Kolossa-Gehring M (2017) New HBM values for emerging substances, inventory of reference and HBM values in force, and working principles of the German Human Biomonitoring Commission. Int J Hyg Environ Health 220(2 Pt A):152–166. https://doi.org/10.1016/j.ijheh.2016.09.007
Apel C, Joerss H, Ebinghaus R (2018) Environmental occurrence and hazard of organic UV stabilizers and UV filters in the sediment of European North and Baltic Seas. Chemosphere 212:254–261. https://doi.org/10.1016/j.chemosphere.2018.08.105
Balázs A, Krifaton C, Orosz I, Szoboszlay S, Kovács R, Csenki Z, Urbányi B, Kriszt B (2016) Hormonal activity, cytotoxicity and developmental toxicity of UV filters. Ecotoxicol Environ Saf 131:45–53. https://doi.org/10.1016/j.ecoenv.2016.04.037
Balmer ME, Buser H-R, Müller MD, Poiger T (2005) Occurrence of some organic UV filters in wastewater, in surface waters, and in fish from Swiss Lakes. Environ Sci Technol 39(4):953–962. https://doi.org/10.1021/es040055r
Barbosa V, Maulvault AL, Alves RN, Kwadijk C, Kotterman M, Tediosi A, Fernández-Tejedor M, Sloth JJ, Granby K, Rasmussen RR, Robbens J, Witte B de, Trabalón L, Fernandes JO, Cunha SC, Marques A (2018) Effects of steaming on contaminants of emerging concern levels in seafood. Food Chem Toxicol 118:490–504. https://doi.org/10.1016/j.fct.2018.05.047
Bury D, Belov VN, Qi Y, Hayen H, Volmer DA, Brüning T, Koch HM (2018) Determination of urinary metabolites of the emerging UV filter octocrylene by online-SPE-LC-MS/MS. Anal Chem 90(1):944–951. https://doi.org/10.1021/acs.analchem.7b03996
Buser H-R, Balmer ME, Schmid P, Kohler M (2006) Occurrence of UV filters 4-methylbenzylidene camphor and octocrylene in fish from various swiss rivers with inputs from wastewater treatment plants. Environ Sci Technol 40(5):1427–1431. https://doi.org/10.1021/es052088s
Byers JP, Sarver JG (2009) Pharmacokinetic modeling. In: Hacker MP, Messer WS, Bachmann KA (eds) Pharmacology. Principles and practice. Elsevier/Academic Press, Amsterdam, pp 201–277
Calafat AM, Longnecker MP, Koch HM, Swan SH, Hauser R, Goldman LR, Lanphear BP, Rudel RA, Engel SM, Teitelbaum SL, Whyatt RM, Wolff MS (2015) Optimal exposure biomarkers for nonpersistent chemicals in environmental epidemiology. Environ Health Perspect 123(7):A166–A168. https://doi.org/10.1289/ehp.1510041
Chatelain E, Gabard B, Surber C (2003) Skin penetration and sun protection factor of five UV filters: effect of the vehicle. Skin Pharmacol Appl Skin Physiol 16(1):28–35. https://doi.org/10.1159/000068291
Conrad A, Schröter-Kermani C, Hoppe H-W, Rüther M, Pieper S, Kolossa-Gehring M (2017) Glyphosate in German adults—time trend (2001 to 2015) of human exposure to a widely used herbicide. Int J Hyg Environ Health 220(1):8–16. https://doi.org/10.1016/j.ijheh.2016.09.016
Cunha SC, Trabalón L, Jacobs S, Castro M, Fernandez-Tejedor M, Granby K, Verbeke W, Kwadijk C, Ferrari F, Robbens J, Sioen I, Pocurull E, Marques A, Fernandes JO, Domingo JL (2018) UV-filters and musk fragrances in seafood commercialized in Europe Union: occurrence, risk and exposure assessment. Environ Res 161:399–408. https://doi.org/10.1016/j.envres.2017.11.015
European Chemicals Agency CAS no.: 178671-58-4—Substance information. https://echa.europa.eu/de/substance-information/-/substanceinfo/100.102.752. Accessed 10 Dec 2018
European Chemicals Agency ECHA disseminated dossier. https://echa.europa.eu/de/registration-dossier/-/registered-dossier/14858/7/5/2. https://echa.europa.eu/de/registration-dossier/-/registered-dossier/14858/7/9/2. https://echa.europa.eu/de/registration-dossier/-/registered-dossier/14858/7/9/2/?documentUUID=38b5fcf8-c7aa-4eb9-89f5-d89e8a1c26bb. https://echa.europa.eu/de/registration-dossier/-/registered-dossier/14858/7/9/3
European Chemicals Agency ECHA disseminated dossier—sub chronic toxicity study in rats. https://echa.europa.eu/de/registration-dossier/-/registered-dossier/14858/7/6/2. Accessed 5 Dec 2018
European Chemicals Agency Etocrilene—Substance information. https://echa.europa.eu/de/substance-information/-/substanceinfo/100.023.663. Accessed 5 Dec 2018
European Chemicals Agency Octocrilene—Substance Information. https://echa.europa.eu/de/substance-information/-/substanceinfo/100.025.683. Accessed 9 Aug 2017
European Chemicals Agency PACT—RMOA and hazard assessment activities—Octocrylene—ECHA. https://echa.europa.eu/de/addressing-chemicals-of-concern/substances-of-potential-concern/pact/-/substance-rev/8118/del/200/col/synonymDynamicField_3413/type/desc/pre/2/view. Accessed 01 Oct 2018
European Commission (2011) Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food: Reg. (EU) 10/2011
European Parliament and the Council (2009) Regulation (EC) No 1223/2009 of the European Parliament and the Council of 30 November 2009 on cosmetic products: Reg. (EC) 1223/2009
Fisch K, Waniek JJ, Schulz-Bull DE (2017) Occurrence of pharmaceuticals and UV-filters in riverine run-offs and waters of the German Baltic Sea. Mar Pollut Bull 124(1):388–399. https://doi.org/10.1016/j.marpolbul.2017.07.057
Forestier S (2008) Rationale for sunscreen development. J Am Acad Dermatol 58(5 Suppl 2):S133–S138. https://doi.org/10.1016/j.jaad.2007.05.047
Greinert R, Vries E de, Erdmann F, Espina C, Auvinen A, Kesminiene A, Schüz J (2015) European Code against Cancer 4th Edition: Ultraviolet radiation and cancer. Cancer Epidemiol 39(Suppl 1):S75–S83. https://doi.org/10.1016/j.canep.2014.12.014
Groot AC de, Roberts DW (2014) Contact and photocontact allergy to octocrylene: a review. Contact Dermat 70(4):193–204. https://doi.org/10.1111/cod.12205
Haines DA, Saravanabhavan G, Werry K, Khoury C (2017) An overview of human biomonitoring of environmental chemicals in the Canadian Health Measures Survey: 2007–2019. Int J Hyg Environ Health 220(2 Pt A):13–28. https://doi.org/10.1016/j.ijheh.2016.08.002
Harper HA, Rodwell VW, Mayes PA (1977) Review of physiological chemistry. Lange Medical Publications, Los Altos
Hayden CGJ, Cross SE, Anderson C, Saunders NA, Roberts MS (2005) Sunscreen penetration of human skin and related keratinocyte toxicity after topical application. Skin Pharmacol Physiol 18(4):170–174. https://doi.org/10.1159/000085861
He K, Timm A, Blaney L (2017) Simultaneous determination of UV-filters and estrogens in aquatic invertebrates by modified quick, easy, cheap, effective, rugged, and safe extraction and liquid chromatography tandem mass spectrometry. J Chromatogr A 1509:91–101. https://doi.org/10.1016/j.chroma.2017.06.039
Kameda Y, Kimura K, Miyazaki M (2011) Occurrence and profiles of organic sun-blocking agents in surface waters and sediments in Japanese rivers and lakes. Environ Pollut (Barking Essex: 1987) 159(6):1570–1576. https://doi.org/10.1016/j.envpol.2011.02.055
Karlsson I, Vanden Broecke K, Mårtensson J, Goossens A, Börje A (2011) Clinical and experimental studies of octocrylene‘s allergenic potency. Contact Dermat 64(6):343–352. https://doi.org/10.1111/j.1600-0536.2011.01899.x
Kerr AC (2011) A survey of the availability of sunscreen filters in the UK. Clin Exp Dermatol 36(5):541–543. https://doi.org/10.1111/j.1365-2230.2010.04007.x
Koch HM, Schütze A, Pälmke C, Angerer J, Brüning T (2013) Metabolism of the plasticizer and phthalate substitute diisononyl-cyclohexane-1,2-dicarboxylate (DINCH(®)) in humans after single oral doses. Arch Toxicol 87(5):799–806. https://doi.org/10.1007/s00204-012-0990-4
Koch HM, Rüther M, Schütze A, Conrad A, Pälmke C, Apel P, Brüning T, Kolossa-Gehring M (2017) Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. Int J Hyg Environ Health 220(2 Pt A):130–141. https://doi.org/10.1016/j.ijheh.2016.11.003
Kohn MC, Parham F, Masten SA, Portier CJ, Shelby MD, Brock JW, Needham LL (2000) Human exposure estimates for phthalates. Environ Health Perspect 108(10):A440–A442. https://doi.org/10.1289/ehp.108-a440b
Kolossa-Gehring M, Fiddicke U, Leng G, Angerer J, Wolz B (2017) New human biomonitoring methods for chemicals of concern—the German approach to enhance relevance. Int J Hyg Environ Health 220(2 Pt A):103–112. https://doi.org/10.1016/j.ijheh.2016.10.012
Kunz PY, Fent K (2006) Multiple hormonal activities of UV filters and comparison of in vivo and in vitro estrogenic activity of ethyl-4-aminobenzoate in fish. Aquat Toxicol (Amsterdam Netherlands) 79(4):305–324. https://doi.org/10.1016/j.aquatox.2006.06.016
Langford KH, Reid MJ, Fjeld E, Øxnevad S, Thomas KV (2015) Environmental occurrence and risk of organic UV filters and stabilizers in multiple matrices in Norway. Environ Int 80:1–7. https://doi.org/10.1016/j.envint.2015.03.012
Lessmann F, Bury D, Weiss T, Hayen H, Brüning T, Koch HM (2018) De-novo identification of specific exposure biomarkers of the alternative plasticizer di(2-ethylhexyl) terephthalate (DEHTP) after low oral dosage to male volunteers by HPLC-Q-Orbitrap-MS. Biomarkers 23(2):196–206. https://doi.org/10.1080/1354750X.2017.1410856
Lopardo L, Adams D, Cummins A, Kasprzyk-Hordern B (2018) Verifying community-wide exposure to endocrine disruptors in personal care products - In quest for metabolic biomarkers of exposure via in vitro studies and wastewater-based epidemiology. Water Res 143:117–126. https://doi.org/10.1016/j.watres.2018.06.028
Mandaric L, Diamantini E, Stella E, Cano-Paoli K, Valle-Sistac J, Molins-Delgado D, Bellin A, Chiogna G, Majone B, Diaz-Cruz MS, Sabater S, Barcelo D, Petrovic M (2017) Contamination sources and distribution patterns of pharmaceuticals and personal care products in Alpine rivers strongly affected by tourism. Sci Total Environ 590–591:484–494. https://doi.org/10.1016/j.scitotenv.2017.02.185
Manová E, Goetz N von, Hauri U, Bogdal C, Hungerbühler K (2013) Organic UV filters in personal care products in Switzerland: a survey of occurrence and concentrations. Int J Hyg Environ Health 216(4):508–514. https://doi.org/10.1016/j.ijheh.2012.08.003
Mizukawa A, Molins-Delgado D, Azevedo JCR de, Fernandes CVS, Díaz-Cruz S, Barceló D (2017) Sediments as a sink for UV filters and benzotriazoles: the case study of Upper Iguaçu watershed, Curitiba (Brazil). Environ Sci Pollut Res Int 24(22):18284–18294. https://doi.org/10.1007/s11356-017-9472-9
Molins-Delgado D, Muñoz R, Nogueira S, Alonso MB, Torres JP, Malm O, Ziolli RL, Hauser-Davis RA, Eljarrat E, Barceló D, Díaz-Cruz MS (2018) Occurrence of organic UV filters and metabolites in lebranche mullet (Mugil liza) from Brazil. Sci Total Environ 618:451–459. https://doi.org/10.1016/j.scitotenv.2017.11.033
Moos RK, Angerer J, Dierkes G, Brüning T, Koch HM (2016) Metabolism and elimination of methyl, iso- and n-butyl paraben in human urine after single oral dosage. Arch Toxicol 90(11):2699–2709. https://doi.org/10.1007/s00204-015-1636-0
Needham LL, Calafat AM, Barr DB (2007) Uses and issues of biomonitoring. Int J Hyg Environ Health 210(3–4):229–238. https://doi.org/10.1016/j.ijheh.2006.11.002
Negreira N, Rodríguez I, Rubí E, Cela R (2009) Determination of selected UV filters in indoor dust by matrix solid-phase dispersion and gas chromatography-tandem mass spectrometry. J Chromatogr A 1216(31):5895–5902. https://doi.org/10.1016/j.chroma.2009.06.020
Peng X, Fan Y, Jin J, Xiong S, Liu J, Tang C (2017) Bioaccumulation and biomagnification of ultraviolet absorbents in marine wildlife of the Pearl River Estuarine, South China Sea. Environ Pollut (Barking, Essex: 1987) 225:55–65. https://doi.org/10.1016/j.envpol.2017.03.035
Picot-Groz M, Fenet H, Martinez Bueno MJ, Rosain D, Gomez E (2018) Diurnal variations in personal care products in seawater and mussels at three Mediterranean coastal sites. Environ Sci Pollut Res Int 25(9):9051–9059. https://doi.org/10.1007/s11356-017-1100-1
Pintado-Herrera MG, Combi T, Corada-Fernández C, González-Mazo E, Lara-Martín PA (2017) Occurrence and spatial distribution of legacy and emerging organic pollutants in marine sediments from the Atlantic coast (Andalusia, SW Spain). Sci Total Environ 605–606:980–994. https://doi.org/10.1016/j.scitotenv.2017.06.055
Plagellat C, Kupper T, Furrer R, Alencastro LF de, Grandjean D, Tarradellas J (2006) Concentrations and specific loads of UV filters in sewage sludge originating from a monitoring network in Switzerland. Chemosphere 62(6):915–925. https://doi.org/10.1016/j.chemosphere.2005.05.024
Potard G (1999) Quantitative HPLC analysis of sunscreens and caffeine during in vitro percutaneous penetration studies. Int J Pharm 189(2):249–260. https://doi.org/10.1016/S0378-5173(99)00258-6
Potard G, Laugel C, Schaefer H, Marty JP (2000) The stripping technique: In vitro absorption and penetration of five UV filters on excised fresh human skin. Skin Pharmacol Appl Skin Physiol 13(6):336–344. https://doi.org/10.1159/000029941
Rodil R, Schrader S, Moeder M (2009) Non-porous membrane-assisted liquid–liquid extraction of UV filter compounds from water samples. J Chromatogr A 1216(24):4887–4894. https://doi.org/10.1016/j.chroma.2009.04.042
Ruppert L, Ofenloch R, Surber C, Diepgen T (2016) Occupational risk factors for skin cancer and the availability of sun protection measures at German outdoor workplaces. Int Arch Occup Environ Health 89(6):1009–1015. https://doi.org/10.1007/s00420-016-1138-2
Salthammer T, Zhang Y, Mo J, Koch HM, Weschler CJ (2018) Assessing human exposure to organic pollutants in the indoor environment. Angew Chem (International ed. English) 57(38):12228–12263. https://doi.org/10.1002/anie.201711023
Sánchez Rodríguez A, Rodrigo Sanz M, Betancort Rodríguez JR (2015) Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands). An approach to environmental risk assessment. Chemosphere 131:85–90. https://doi.org/10.1016/j.chemosphere.2015.02.054
Schindler BK, Esteban M, Koch HM, Castano A, Koslitz S, Cañas A, Casteleyn L, Kolossa-Gehring M, Schwedler G, Schoeters G, Hond ED, Sepai O, Exley K, Bloemen L, Horvat M, Knudsen LE, Joas A, Joas R, Biot P, Aerts D, Lopez A, Huetos O, Katsonouri A, Maurer-Chronakis K, Kasparova L, Vrbík K, Rudnai P, Naray M, Guignard C, Fischer ME, Ligocka D, Janasik B, Reis MF, Namorado S, Pop C, Dumitrascu I, Halzlova K, Fabianova E, Mazej D, Tratnik JS, Berglund M, Jönsson B, Lehmann A, Crettaz P, Frederiksen H, Nielsen F, McGrath H, Nesbitt I, Cremer K de, Vanermen G, Koppen G, Wilhelm M, Becker K, Angerer J (2014) The European COPHES/DEMOCOPHES project: towards transnational comparability and reliability of human biomonitoring results. Int J Hyg Environ Health 217(6):653–661. https://doi.org/10.1016/j.ijheh.2013.12.002
Schlumpf M, Kypke K, Vökt CC, Birchler M, Durrer S, Faass O, Ehnes C, Fuetsch M, Gaille C, Henseler M, Hofkamp L, Maerkel K, Reolon S, Zenker A, Timms B, Tresguerres JAF, Lichtensteigera W (2008) Endocrine active uv filters: developmental toxicity and exposure through breast milk. Chimia 62(5):345–351. https://doi.org/10.2533/chimia.2008.345
Schlumpf M, Kypke K, Wittassek M, Angerer J, Mascher H, Mascher D, Vökt C, Birchler M, Lichtensteiger W (2010) Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: correlation of UV filters with use of cosmetics. Chemosphere 81(10):1171–1183. https://doi.org/10.1016/j.chemosphere.2010.09.079
Schoeters G, Govarts E, Bruckers L, Den Hond E, Nelen V, Henauw S de, Sioen I, Nawrot TS, Plusquin M, Vriens A, Covaci A, Loots I, Morrens B, Coertjens D, van Larebeke N, Craemer S de, Croes K, Lambrechts N, Colles A, Baeyens W (2017) Three cycles of human biomonitoring in Flanders—time trends observed in the Flemish Environment and Health Study. Int J Hyg Environ Health 220(2 Pt A):36–45. https://doi.org/10.1016/j.ijheh.2016.11.006
Schütze A, Otter R, Modick H, Langsch A, Brüning T, Koch HM (2017) Additional oxidized and alkyl chain breakdown metabolites of the plasticizer DINCH in urine after oral dosage to human volunteers. Arch Toxicol 91(1):179–188. https://doi.org/10.1007/s00204-016-1688-9
Schwedler G, Joas A, Calafat AM, Haines D, Nakayama S, Wolz B, Kolossa-Gehring M (2017) 2nd international conference on human biomonitoring, Berlin 2016. Int J Hyg Environ Health 220(2 Pt A):1–2. https://doi.org/10.1016/j.ijheh.2017.01.004
Shaath NA (2010) Ultraviolet filters. Photochem Photobiol Sci 9(4):464–469. https://doi.org/10.1039/b9pp00174c
Silva MJ, Furr J, Preau JL, Samandar E, Gray LE, Calafat AM (2012) Identification of potential biomarkers of exposure to di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH), an alternative for phthalate plasticizers. J Expo Sci Environ Epidemiol 22(2):204–211. https://doi.org/10.1038/jes.2011.43
Stien D, Clergeaud F, Rodrigues AMS, Lebaron K, Pillot R, Romans P, Fagervold S, Lebaron P (2018) Metabolomics reveal that octocrylene accumulates in Pocillopora damicornis tissues as fatty acid conjugates and triggers coral cell mitochondrial dysfunction. Anal Chem. https://doi.org/10.1021/acs.analchem.8b04187
Strajhar P, Tonoli D, Jeanneret F, Imhof RM, Malagnino V, Patt M, Kratschmar DV, Boccard J, Rudaz S, Odermatt A (2017) Steroid profiling in H295R cells to identify chemicals potentially disrupting the production of adrenal steroids. Toxicology 381:51–63. https://doi.org/10.1016/j.tox.2017.02.010
The European Multicentre Photopatch Test Study Taskforce (2012) A European multicentre photopatch test study. Br J Dermatol 166(5):1002–1009. https://doi.org/10.1111/j.1365-2133.2012.10857.x
Tsui MMP, Lam JCW, Ng TY, Ang PO, Murphy MB, Lam PKS (2017) Occurrence, distribution, and fate of organic UV filters in coral communities. Environ Sci Technol 51(8):4182–4190. https://doi.org/10.1021/acs.est.6b05211
U.S. Food & Drug Administration. 21 Code of Federal Regulations § 352.10.: 21 C.F.R. § 352.10 (revised as of 1 April 2018)
Ulrich N, Bury D, Koch HM, Rüther M, Weber T, Käfferlein H-U, Weiss T, Brüning T, Kolossa-Gehring M (2018) Metabolites of the alkyl pyrrolidone solvents NMP and NEP in 24-h urine samples of the German Environmental Specimen Bank from 1991 to 2014. Int Arch Occup Environ Health. https://doi.org/10.1007/s00420-018-1347-y
Uter W, Gonçalo M, Yazar K, Kratz E-M, Mildau G, Lidén C (2014) Coupled exposure to ingredients of cosmetic products: III. Ultraviolet filters. Contact Dermat 71(3):162–169. https://doi.org/10.1111/cod.12245
Uter W, Lessmann H, Geier J (2017) Is octocrylene a frequent contact allergen? Contact Dermat 77(2):127–128. https://doi.org/10.1111/cod.12761
Vila M, Llompart M, Garcia-Jares C, Homem V, Dagnac T (2018) Development and optimization of a solid-phase microextraction gas chromatography-tandem mass spectrometry methodology to analyse ultraviolet filters in beach sand. J Chromatogr A 1564:59–68. https://doi.org/10.1016/j.chroma.2018.06.016
Wahie S, Lloyd JJ, Farr PM (2007) Sunscreen ingredients and labelling: a survey of products available in the UK. Clin Exp Dermatol 32(4):359–364. https://doi.org/10.1111/j.1365-2230.2007.02404.x
Wang SQ, Tanner PR, Lim HW, Nash JF (2013) The evolution of sunscreen products in the United States—a 12-year cross sectional study. Photochem Photobiol Sci 12(1):197–202. https://doi.org/10.1039/c2pp25112d
Acknowledgements
The presented study and development of the analytical method applied herein are part of a large-scale 10-year project on the advancement of human biomonitoring in Germany. This project is a cooperation agreed in 2010 between the German Federal Ministry for the Environment, Nature Conservation and Nuclear Safety (BMU) and the German Chemical Industry Association (VCI-Verband der Chemischen Industrie e.V.) and is managed by the German Environment Agency (UBA). Experts from governmental scientific authorities, industry and science provide advice in the selection of substances to be investigated and during method development. Analytical method development and the human metabolism study were financed by the Chemie Wirtschaftsförderungsgesellschaft mbH, while the first application of the novel methodology in a larger population study will be financed by the German Environment Agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The participation of Edgar Leibold as co-author was conducted as part of his employment responsibilities with BASF SE, a manufacturer of octocrylene. The interpretation and views expressed in this manuscript are not necessarily those of the co-author's employer.
Ethical approval
The study was carried out in accordance with the Code of Ethics of the World Medical Association (1964 Declaration of Helsinki and later amendments) and has been approved by the ethical review board of the medical faculty of the Ruhr-University Bochum (IRB Reg. No.: 4288-12). The study design was presented to the volunteers in written form and written informed consent was obtained from each individual participant.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bury, D., Modick-Biermann, H., Leibold, E. et al. Urinary metabolites of the UV filter octocrylene in humans as biomarkers of exposure. Arch Toxicol 93, 1227–1238 (2019). https://doi.org/10.1007/s00204-019-02408-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-019-02408-7