Abstract
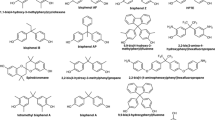
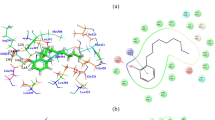
The binding interactions of bisphenol A (BPA) and its halogenated derivatives (halogenated BPAs) to human estrogen receptor α ligand binding domain (hERα-LBD) was investigated using a combined in vitro and in silico approach. First, the recombinant hERα-LBD was prepared as a soluble protein in Escherichia coli BL21(DE3)pLysS. A native fluorescent phytoestrogen, coumestrol, was employed as tracer for the fluorescence polarization assay. The results of the in vitro binding assay showed that bisphenol compounds could bind to hERα-LBD as the affinity ligands. All the tested halogenated BPAs exhibited weaker receptor binding than BPA, which might be explained by the steric effect of substituents. Molecular docking studies elucidated that the halogenated BPAs adopted different conformations in the flexible hydrophobic ligand binding pocket (LBP), which is mainly dependent on their distinct halogenation patterns. The compounds with halogen substituents on the phenolic rings and on the bridging alkyl moiety acted as agonists and antagonists for hERα, respectively. Interestingly, all the compounds in the agonist conformation of hERα formed a hydrogen bond with His524, while the compounds in the antagonist conformation formed a hydrogen bond with Thr347. These docking results suggested a pivotal role of His524/Thr347 in maintaining the hERα structure in the biologically active agonist/antagonist conformation. Comparison of the calculated binding energies vs. experimental binding affinities yielded a good correlation, which might be applicable for the structure-based design of novel bisphenol compounds with reduced toxicities and for environmental risk assessment. In addition, based on hERα-LBD as a recognition element, the proposed fluorescence polarization assay may offer an alternative to chromatographic techniques for the multi-residue determination of bisphenol compounds.






Similar content being viewed by others
References
Andra SS, Charisiadis P, Arora M, van Vliet-Ostaptchouk JV, Makris KC (2015) Biomonitoring of human exposures to chlorinated derivatives and structural analogs of bisphenol A. Environ Int 85:352–379. https://doi.org/10.1016/j.envint.2015.09.011
Atkinson JC, Diamond F, Eichmiller F, Selwitz R, Jones G (2002) Stability of bisphenol A, triethylene-glycol dimethacrylate, and bisphenol A dimethacrylate in whole saliva. Dent Mater 18(2):128–135. https://doi.org/10.1016/S0109-5641(01)00031-8
Audebert M, Dolo L, Perdu E, Cravedi JP, Zalko D (2011) Use of the γH2AX assay for assessing the genotoxicity of bisphenol A and bisphenol F in human cell lines. Arch Toxicol 85(11):1463–1473. https://doi.org/10.1007/s00204-011-0721-2
Bruning JB, Parent AA, Gil G et al (2010) Coupling of receptor conformation and ligand orientation determine graded activity. Nat Chem Biol 6(11):837–843. https://doi.org/10.1038/nchembio.451
Chen D, Kannan K, Tan H et al (2016) Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity—a review. Environ Sci Technol 50(11):5438–5453. https://doi.org/10.1021/acs.est.5b05387
Colnot T, Kacew S, Dekant W (2014) Mammalian toxicology and human exposures to the flame retardant 2,2′,6,6′-tetrabromo-4,4′-isopropylidenediphenol (TBBPA): implications for risk assessment. Arch Toxicol 88(3):553–573. https://doi.org/10.1007/s00204-013-1180-8
Deiminiat B, Rounaghi GH, Arbab-Zavar MH, Razavipanah I (2017) A novel electrochemical aptasensor based on f-MWCNTs/AuNPs nanocomposite for label-free detection of bisphenol A. Sens Actuators B Chem 242(Supplement C):158–166. https://doi.org/10.1016/j.snb.2016.11.041
Delfosse V, Grimaldi M, Pons J-L et al (2012) Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc Natl Acad Sci USA 109(37):14930–14935. https://doi.org/10.1073/pnas.1203574109
Dietrich R, Hengstler D JG (2016) From bisphenol A to bisphenol F and a ban of mustard due to chronic low-dose exposures? Arch Toxicol 90(2):489–491. https://doi.org/10.1007/s00204-016-1671-5
Ekena K, Weis KE, Katzenellenbogen JA, Katzenellenbogen BS (1996) Identification of amino acids in the hormone binding domain of the human estrogen receptor important in estrogen binding. J Biol Chem 271(33):20053–20059. https://doi.org/10.1074/jbc.271.33.20053
Feng Y, Jiao Z, Shi J, Li M, Guo Q, Shao B (2016) Effects of bisphenol analogues on steroidogenic gene expression and hormone synthesis in H295R cells. Chemosphere 147:9–19. https://doi.org/10.1016/j.chemosphere.2015.12.081
Fernandez MF, Arrebola JP, Taoufiki J et al (2007) Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod Toxicol 24(2):259–264. https://doi.org/10.1016/j.reprotox.2007.06.007
Fillon M (2012) Getting it right: BPA and the difficulty proving environmental cancer risks. J Natl Cancer Inst 104(9):652–655. https://doi.org/10.1093/jnci/djs237
Geens T, Goeyens L, Kannan K, Neels H, Covaci A (2012) Levels of bisphenol-A in thermal paper receipts from Belgium and estimation of human exposure. Sci Total Environ 435–436:30–33. https://doi.org/10.1016/j.scitotenv.2012.07.001
Gosavi RA, Knudsen GA, Birnbaum LS, Pedersen LC (2013) Mimicking of estradiol binding by flame retardants and their metabolites: a crystallographic analysis. Environ Health Perspect 121(10):1194–1199. https://doi.org/10.1289/ehp.1306902
He Y, Miao M, Herrinton LJ et al (2009) Bisphenol A levels in blood and urine in a Chinese population and the personal factors affecting the levels. Environ Res 109(5):629–633. https://doi.org/10.1016/j.envres.2009.04.003
Hewitt SC, Korach KS (2011) Estrogenic activity of bisphenol A and 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE) demonstrated in mouse uterine gene profiles. Environ Health Perspect 119(1):63–70. https://doi.org/10.1289/ehp.1002347
Hong H, Kohli K, Trivedi A, Johnson DL, Stallcup MR (1996) GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc Natl Acad Sci USA 93(10):4948–4952. https://doi.org/10.1073/pnas.93.10.4948
Jia M, Dahlman-Wright K, Gustafsson J-Å (2015) Estrogen receptor alpha and beta in health and disease. Best Pract Res Clin Endocrinol Metab 29(4):557–568. https://doi.org/10.1016/j.beem.2015.04.008
Kitamura S, Suzuki T, Sanoh S et al (2005) Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci 84(2):249–259. https://doi.org/10.1093/toxsci/kfi074
Lane RF, Adams CD, Randtke SJ, Carter RE (2015) Bisphenol diglycidyl ethers and bisphenol A and their hydrolysis in drinking water. Water Res 72:331–339. https://doi.org/10.1016/j.watres.2014.09.043
Lee HJ, Chattopadhyay S, Gong E-Y, Ahn RS, Lee K (2003) Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci 75(1):40–46. https://doi.org/10.1093/toxsci/kfg150
Lin W, Huang J, Liao X et al (2016) Neo-tanshinlactone selectively inhibits the proliferation of estrogen receptor positive breast cancer cells through transcriptional down-regulation of estrogen receptor alpha. Pharmacol Res 111:849–858. https://doi.org/10.1016/j.phrs.2016.07.044
Maćczak A, Cyrkler M, Bukowska B, Michałowicz J (2016) Eryptosis-inducing activity of bisphenol A and its analogs in human red blood cells (in vitro study). J Hazard Mater 307:328–335. https://doi.org/10.1016/j.jhazmat.2015.12.057
Maffini MV, Rubin BS, Sonnenschein C, Soto AM (2006) Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol 254–255:179–186. https://doi.org/10.1016/j.mce.2006.04.033
Matthews JB, Twomey K, Zacharewski TR (2001) In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors α and β. Chem Res Toxicol 14(2):149–157. https://doi.org/10.1021/tx0001833
Meeker JD, Ehrlich S, Toth TL et al (2010) Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod Toxicol 30(4):532–539. https://doi.org/10.1016/j.reprotox.2010.07.005
Molina-Molina J-M, Amaya E, Grimaldi M et al (2013) In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol Appl Pharmacol 272(1):127–136. https://doi.org/10.1016/j.taap.2013.05.015
Moraes FC, Silva TA, Cesarino I, Machado SAS (2013) Effect of the surface organization with carbon nanotubes on the electrochemical detection of bisphenol A. Sens Actuators B Chem 177(Supplement C):14–18 https://doi.org/10.1016/j.snb.2012.10.128
Murphy RB, Repasky MP, Greenwood JR et al (2016) WScore: a flexible and accurate treatment of explicit water molecules in ligand–receptor docking. J Med Chem 59(9):4364–4384. https://doi.org/10.1021/acs.jmedchem.6b00131
Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B (2000) Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor α and estrogen receptor β. Proc Natl Acad Sci USA 97(21):11603–11608. https://doi.org/10.1073/pnas.97.21.11603
Nagy L, Schwabe JW (2004) Mechanism of the nuclear receptor molecular switch. Trends Biochem Sci 29(6):317–324. https://doi.org/10.1016/j.tibs.2004.04.006
Pike AC (2006) Lessons learnt from structural studies of the oestrogen receptor. Best Pract Res Clin Endocrinol Metab 20(1):1–14. https://doi.org/10.1016/j.beem.2005.09.002
Riu A, Grimaldi M, le Maire A et al (2011) Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ Health Perspect 119(9):1227–1232. https://doi.org/10.1289/ehp.1003328
Rochester JR, Bolden AL (2015) Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect 123(7):643–650. https://doi.org/10.1289/ehp.1408989
Routledge EJ, White R, Parker MG, Sumpter JP (2000) Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) α and ERβ. J Biol Chem 275(46):35986–35993. https://doi.org/10.1074/jbc.m006777200
Sáiz J, Gómara B (2017) Evaluation of endocrine disrupting compounds migration in household food containers under domestic use conditions. J Agric Food Chem 65(31):6692–6700. https://doi.org/10.1021/acs.jafc.7b02479
Shiau AK, Barstad D, Loria PM et al (1998) The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95(7):927–937. https://doi.org/10.1016/S0092-8674(00)81717-1
Song S, Song M, Zeng L et al (2014) Occurrence and profiles of bisphenol analogues in municipal sewage sludge in China. Environ Pollut 186:14–19. https://doi.org/10.1016/j.envpol.2013.11.023
Srinivasan S, Nwachukwu JC, Bruno NE et al (2017) Full antagonism of the estrogen receptor without a prototypical ligand side chain. Nat Chem Biol 13(1):111–118. https://doi.org/10.1038/nchembio.2236
Sui Y, Ai N, Park S-H et al (2012) Bisphenol A and its analogues activate human pregnane X receptor. Environ Health Perspect 120(3):399–405. https://doi.org/10.1289/ehp.1104426
Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV (2007) Human exposure to bisphenol A (BPA). Reprod Toxicol 24(2):139–177. https://doi.org/10.1016/j.reprotox.2007.07.010
Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM (2009) Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 30(1):75–95. https://doi.org/10.1210/er.2008-0021
Vitku J, Chlupacova T, Sosvorova L et al (2015) Development and validation of LC-MS/MS method for quantification of bisphenol A and estrogens in human plasma and seminal fluid. Talanta 140:62–67. https://doi.org/10.1016/j.talanta.2015.03.013
Völkel W, Kiranoglu M, Fromme H (2008) Determination of free and total bisphenol A in human urine to assess daily uptake as a basis for a valid risk assessment. Toxico Lett 179(3):155–162. https://doi.org/10.1016/j.toxlet.2008.05.002
Watson CS, Bulayeva NN, Wozniak AL, Finnerty CC (2005) Signaling from the membrane via membrane estrogen receptor-α: estrogens, xenoestrogens, and phytoestrogens. Steroids 70(5–7):364–371. https://doi.org/10.1016/j.steroids.2005.03.002
Zhang J, Zhang T, Guan T et al (2017a) Spectroscopic and molecular modeling approaches to investigate the interaction of bisphenol A, bisphenol F and their diglycidyl ethers with PPARα. Chemosphere 180:253–258. https://doi.org/10.1016/j.chemosphere.2017.04.034
Zhang J, Zhang T, Guan T, Yu H, Li T (2017b) In vitro and in silico assessment of the structure-dependent binding of bisphenol analogues to glucocorticoid receptor. Anal Bioanal Chem 409(8):2239–2246. https://doi.org/10.1007/s00216-016-0168-7
Zoeller RT, Bansal R, Parris C (2005) Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology 146(2):607–612. https://doi.org/10.1210/en.2004-1018
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2017YFD0300303 and 2017YFD0300603), the National Natural Science Foundation of China (31601534), the Project funded by China Postdoctoral Science Foundation (2017M621213), and the Agricultural Science and Technology Innovation Program of Jilin Province (CXGC2017JQ006 and CXGC2017JQ010).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical standards
The manuscript does not contain clinical studies or participant data.
Rights and permissions
About this article
Cite this article
Zhang, J., Li, T., Wang, T. et al. Estrogenicity of halogenated bisphenol A: in vitro and in silico investigations. Arch Toxicol 92, 1215–1223 (2018). https://doi.org/10.1007/s00204-017-2127-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-017-2127-2