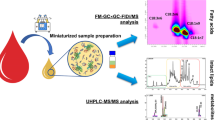
Abstract
Lipidomics is the area of study dedicated to the comprehensive analysis and characterization of the functions and metabolism of lipids in biological samples. One of the most comprehensively studied classes of lipids is polyunsaturated fatty acids (PUFAs). Eicosanoids are a series of bioactive lipid mediators that are metabolized from PUFAs and generated majorly from the precursor arachidonic acid. This study identified the profiles of target eicosanoids after acute exposure to arsenic. The principle objective was to determine and validate 10 eicosanoids in mouse serum using on-line solid-phase extraction integrated with liquid chromatography–electrospray tandem mass spectrometry. Intra-day and inter-day repeatability was 82.4–119.2 and 86.7–124.4%, respectively. The limit of detection and limit of quantification were from 0.003 to 0.288 ng/mL and from 0.009 to 0.962 ng/mL, respectively. The levels of 7 of the 10 eicosanoids—namely 8-isoPGF2α, PGF2α, PGE2, 13(s)-HODE, 15(s)-HETE, 12(s)-HETE, and 5(s)-HETE—in mouse serum significantly and dose-dependently increased after arsenic exposure compared with the levels in the vehicle control group. To our knowledge, this is the first study to quantify eicosanoids in mouse serum. This approach provides simple sample preparation, small sample volumes, and a precise and accurate method for determining changes in the profile of these eicosanoids in mouse serum after acute exposure to arsenic.



Similar content being viewed by others
References
Abrial C, Grassin-Delyle S, Salvator H, Brollo M, Naline E, Devillier P (2015) 15-Lipoxygenases regulate the production of chemokines in human lung macrophages. Br J Pharmacol 172:4319–4330. doi:10.1111/bph.13210
Anwar-Mohamed A, El-Sherbeni AA, Kim SH, Althurwi HN, Zordoky BN, El-Kadi AO (2012) Acute arsenic toxicity alters cytochrome P450 and soluble epoxide hydrolase and their associated arachidonic acid metabolism in C57Bl/6 mouse heart. Xenobiot Fate Foreign Compd Biol Syst 42:1235–1247. doi:10.3109/00498254.2012.693971
Anwar-Mohamed A, El-Sherbeni A, Kim SH (2013) Acute arsenic treatment alters cytochrome P450 expression and arachidonic acid metabolism in lung, liver and kidney of C57Bl/6 mice. Xenobiot Fate Foreign Compd Biol Syst 43:719–729. doi:10.3109/00498254.2012.754113
Anwar-Mohamed A, Elshenawy OH, El-Sherbeni AA, Abdelrady M, El-Kadi AOS (2014) Acute arsenic treatment alters arachidonic acid and its associated metabolite levels in the brain of C57Bl/6 mice. Can J Physiol Pharmacol 92:693–702. doi:10.1139/cjpp-2014-0136
Astarita G, Kendall AC, Dennis EA, Nicolaou A (2015) Targeted lipidomic strategies for oxygenated metabolites of polyunsaturated fatty acids. Biochimica et Biophysica Acta (BBA)–Mol Cell Biol Lipids 1851:456–468. doi:10.1016/j.bbalip.2014.11.012
Bashir S, Sharma Y, Irshad M (2006) Arsenic induced apoptosis in rat liver following repeated 60 days exposure. Toxicology 217:63–70. doi:10.1016/j.tox.2005.08.023
Briskey DR, Wilson GR, Fassett RG, Coombes JS (2014) Optimized method for quantification of total F2-isoprostanes using gas chromatography–tandem mass spectrometry. J Pharm Biomed Anal 90:161–166. doi:10.1016/j.jpba.2013.11.028
Chen CY, Jhou YT, Lee HL, Lin YW (2016) Simultaneous, rapid, and sensitive quantification of 8-hydroxy-2′-deoxyguanosine and cotinine in human urine by on-line solid-phase extraction LC-MS/MS: correlation with tobacco exposure biomarkers NNAL. Anal Bioanal Chem 408:6295–6306. doi:10.1007/s00216-016-9741-3
Chiang WC, Chen CY, Lee TC, Lee HL, Lin YW (2015) Fast and simple screening for the simultaneous analysis of seven metabolites derived from five volatile organic compounds in human urine using on-line solid-phase extraction coupled with liquid chromatography-tandem mass spectrometry. Talanta 132:469–478. doi:10.1016/j.talanta.2014.09.029
Cimen I, Astarci E, Banerjee S (2011) 15-lipoxygenase-1 exerts its tumor suppressive role by inhibiting nuclear factor-kappa B via activation of PPAR gamma. J Cell Biochem 112:2490–2501. doi:10.1002/jcb.23174
Cooke MS, Evans MD, Dove R et al (2005) DNA repair is responsible for the presence of oxidatively damaged DNA lesions in urine. Mutat Res/Fundam Mol Mech Mutagen 574:58–66. doi:10.1016/j.mrfmmm.2005.01.022
de Zwart LL, Meerman JHN, Commandeur JNM, Vermeulen NPE (1999) Biomarkers of free radical damage: Applications in experimental animals and in humans. Free Radical Biol Med 26:202–226. doi:10.1016/S0891-5849(98)00196-8
Ding X-Z, Tong WG, Adrian TE (2001) 12-lipoxygenase metabolite 12(S)-HETE stimulates human pancreatic cancer cell proliferation via protein tyrosine phosphorylation and ERK activation. Int J Cancer 94:630–636. doi:10.1002/ijc.1527
Dutta K, Prasad P, Sinha D (2015) Chronic low level arsenic exposure evokes inflammatory responses and DNA damage. Int J Hyg Environ Health 218:564–574. doi:10.1016/j.ijheh.2015.06.003
Ferreiro-Vera C, Mata-Granados JM, Priego-Capote F, Quesada-Gómez JM, Luque de Castro MD (2010) Automated targeting analysis of eicosanoid inflammation biomarkers in human serum and in the exometabolome of stem cells by SPE–LC–MS/MS. Anal Bioanal Chem 399:1093–1103. doi:10.1007/s00216-010-4400-6
Flora SJ (2011) Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med 51:257–281. doi:10.1016/j.freeradbiomed.2011.04.008
Garcia-Sevillano MA, Contreras-Acuna M, Garcia-Barrera T, Navarro F, Gomez-Ariza JL (2014) Metabolomic study in plasma, liver and kidney of mice exposed to inorganic arsenic based on mass spectrometry. Anal Bioanal Chem 406:1455–1469. doi:10.1007/s00216-013-7564-z
Garcia-Sevillano MA, Garcia-Barrera T, Navarro F, Montero-Lobato Z, Gomez-Ariza JL (2015) Shotgun metabolomic approach based on mass spectrometry for hepatic mitochondria of mice under arsenic exposure. Biometals 28:341–351. doi:10.1007/s10534-015-9837-9
Griffiths WJ, Koal T, Wang Y, Kohl M, Enot DP, Deigner HP (2010) Targeted metabolomics for biomarker discovery. Angew Chem 49:5426–5445. doi:10.1002/anie.200905579
Hei TK, Filipic M (2004) Role of oxidative damage in the genotoxicity of arsenic. Free Radical Biol Med 37:574–581. doi:10.1016/j.freeradbiomed.2004.02.003
Hennig R, Kehl T, Noor S (2007) 15-Lipoxygenase-1 production is lost in pancreatic cancer and overexpression of the gene inhibits tumor cell growth. Neoplasia 9:917–926. doi:10.1593/neo.07565
IARC (1987) Arsenic and Arsenic compounds. In: Overall evaluations of carcinogenicity: an updating of IARC monographs volumes 1 to 42. IARC monographs on the evaluation of carcinogenic risk to human, suppl 7. IARC, pp 100–106
IARC (2012) Arsenic and arsenic compounds. Arsenic, metals, fibres, and dusts: a review of human carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans 100C:41–85.
Kohen R, Nyska A (2002) Invited review: oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30:620–650. doi:10.1080/01926230290166724
Kusmartsev S (2012) Enhanced 15-lipoxygenase activity and elevated eicosanoid production in kidney tumor microenvironment contribute to the inflammation and immune suppression. Oncoimmunology 1:249–251. doi:10.4161/onci.1.2.18502
Lagarde M, Calzada C, Jouvène C (2015) Functional fluxolipidomics of polyunsaturated fatty acids and oxygenated metabolites in the blood vessel compartment. Prog Lipid Res 60:41–49. doi:10.1016/j.plipres.2015.10.001
Lin P, Lee HL, Cheng HI, Chen CY, Tsai MH, Liu HJ (2014) Metabolomic profiling of mice urine and serum associated with trans-trans 2, 4-decadienal induced lung lesions by liquid chromatography-mass spectrometry. Anal Bioanal Chem 406:4287–4297. doi:10.1007/s00216-014-7681-3
Liu J, Mazzone PJ, Cata JP (2014) SErum free fatty acid biomarkers of lung cancer. Chest 146:670–679. doi:10.1378/chest.13-2568
Lu W, Bennett BD, Rabinowitz JD (2008) Analytical strategies for LC–MS-based targeted metabolomics. J Chromatogr B 871:236–242. doi:10.1016/j.jchromb.2008.04.031
Ma J, Zhang L, Zhang J (2013) 15-Lipoxygenase-1/15-hydroxyeicosatetraenoic acid promotes hepatocellular cancer cells growth through protein kinase B and heat shock protein 90 complex activation. Int J Biochem Cell Biol 45:1031–1041. doi:10.1016/j.biocel.2013.02.018
Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL (2011) Arsenic exposure and the induction of human cancers. J Toxicol 2011:431287. doi:10.1155/2011/431287
Melstrom LG, Bentrem DJ, Salabat MR (2008) Overexpression of 5-lipoxygenase in colon polyps and cancer and the effect of 5-LOX inhibitors in vitro and in a murine model. Clin Cancer Res 14:6525–6530. doi:10.1158/1078-0432.ccr-07-4631
Milne GL, Gao B, Terry ES, Zackert WE, Sanchez SC (2013) Measurement of F2- isoprostanes and isofurans using gas chromatography–mass spectrometry. Free Radical Biol Med 59:36–44. doi:10.1016/j.freeradbiomed.2012.09.030
Navas-Acien A, Sharrett AR, Silbergeld EK (2005) Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence. Am J Epidemiol 162:1037–1049. doi:10.1093/aje/kwi330
Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E (2006) Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiologic evidence. Environ Health Perspect 114:641–648. doi:10.1289/ehp.8551
Ng DJY, Pasikanti KK, Chan ECY (2011) Trend analysis of metabonomics and systematic review of metabonomics-derived cancer marker metabolites. Metabolomics 7:155–178. doi:10.1007/s11306-010-0250-7
Paige M, Saprito MS, Bunyan DA, Shim YM (2009) HPLC quantification of 5-hydroxyeicosatetraenoic acid in human lung cancer tissues. Biomed Chromatogr 23:817–821. doi:10.1002/bmc.1191
Pidgeon GP, Lysaght J, Krishnamoorthy S (2007) Lipoxygenase metabolism: roles in tumor progression and survival. Cancer Metastasis Rev 26:503–524. doi:10.1007/s10555-007-9098-3
Psychogios N, Hau DD, Peng J (2011) The human serum metabolome. PLoS One 6:e16957. doi:10.1371/journal.pone.0016957
Ramsey KA, Foong RE, Sly PD, Larcombe AN, Zosky GR (2013) Early life arsenic exposure and acute and long-term responses to influenza A infection in mice. Environ Health Perspect 121:1187–1193. doi:10.1289/ehp.1306748
Roberts Ii LJ, Morrow JD (2000) Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radical Biol Med 28:505–513. doi:10.1016/S0891-5849(99)00264-6
Rolim AE, Henrique-Araujo R, Ferraz EG, de Araujo Alves Dultra FK, Fernandez LG (2015) Lipidomics in the study of lipid metabolism: current perspectives in the omic sciences. Gene 554:131–139. doi:10.1016/j.gene.2014.10.039
Serhan CN (1990) [20] High-performance liquid chromatography separation and determination of lipoxins. In: Robert CM, Frank AF (eds) Methods in Enzymology. vol, 187. Academic, pp 167–175
Trufelli H, Palma P, Famiglini G, Cappiello A (2011) An overview of matrix effects in liquid chromatography–mass spectrometry. Mass Spectrom Rev 30:491–509. doi:10.1002/mas.20298
Tsikas D, Suchy MT (2016) Protocols for the measurement of the F2-isoprostane, 15(S)-8-iso-prostaglandin F2alpha, in biological samples by GC-MS or GC-MS/MS coupled with immunoaffinity column chromatography. J Chromatogr B 1019:191–201. doi:10.1016/j.jchromb.2014.12.019
Tsikas D, Rothmann S, Schneider JY (2016a) Development, validation and biomedical applications of stable-isotope dilution GC-MS and GC-MS/MS techniques for circulating malondialdehyde (MDA) after pentafluorobenzyl bromide derivatization: MDA as a biomarker of oxidative stress and its relation to 15(S)-8-iso-prostaglandin F2alpha and nitric oxide (NO). J Chromatogr B 1019:95–111. doi:10.1016/j.jchromb.2015.10.009
Tsikas D, Suchy MT, Todter K, Heeren J, Scheja L (2016b) Utilizing immunoaffinity chromatography (IAC) cross-reactivity in GC-MS/MS exemplified at the measurement of prostaglandin E1 in human plasma using prostaglandin E2-specific IAC columns. J Chromatogr B 1021:101–107. doi:10.1016/j.jchromb.2015.04.026
Unterwurzacher I, Koal T, Bonn GK, Weinberger KM, Ramsay SL (2008) Rapid sample preparation and simultaneous quantitation of prostaglandins and lipoxygenase derived fatty acid metabolites by liquid chromatography-mass spectrometry from small sample volumes. Clin Chem Lab Med 46:1589–1597. doi:10.1515/CCLM.2008.323
Wang D, DuBois RN (2007) Measurement of eicosanoids in cancer tissues methods in enzymology, vol 433. Academic, p 27–50
Wang X, Mu X, Zhang J (2015) Serum metabolomics reveals that arsenic exposure disrupted lipid and amino acid metabolism in rats: a step forward in understanding chronic arsenic toxicity. Metallomics 7:544–552. doi:10.1039/C5MT00002E
Wiktorowska-Owczarek A, Berezinska M, Nowak JZ (2015) PUFAs: Structures, metabolism and functions. Adv Clin Exp Med 24:931–941. doi:10.17219/acem/31243
Yang J, Schmelzer K, Georgi K, Hammock BD (2009) Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem 81:8085–8093. doi:10.1021/ac901282n
Yue H, Strauss KI, Borenstein MR, Barbe MF, Rossi LJ, Jansen SA (2004) Determination of bioactive eicosanoids in brain tissue by a sensitive reversed-phase liquid chromatographic method with fluorescence detection. J Chromatogr B 803:267–277. doi:10.1016/j.jchromb.2003.12.027
Zhang A, Sun H, Wang X (2012) Serum metabolomics as a novel diagnostic approach for disease: a systematic review. Anal Bioanal Chem 404:1239–1245. doi:10.1007/s00216-012-6117-1
Acknowledgements
This study was supported by the National Health Research Institutes (EM-105-PP-02) and the Ministry of Science and Technology, Taiwan (MOST105-2113-M-030-008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, CY., Lin, P., Tsai, MH. et al. Targeted lipidomics profiling of acute arsenic exposure in mice serum by on-line solid-phase extraction stable-isotope dilution liquid chromatography–tandem mass spectrometry. Arch Toxicol 91, 3079–3091 (2017). https://doi.org/10.1007/s00204-017-1937-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-017-1937-6