Abstract
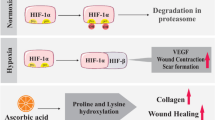
Skin exposure to sulfur mustard (SM) provokes long-term complications in wound healing. Similar to chronic wounds, SM-induced skin lesions are associated with low levels of oxygen in the wound tissue. Normally, skin cells respond to hypoxia by stabilization of the transcription factor hypoxia-inducible factor 1 alpha (HIF-1α). HIF-1α modulates expression of genes including VEGFA, BNIP3, and MMP2 that control processes such as angiogenesis, growth, and extracellular proteolysis essential for proper wound healing. The results of our studies revealed that exposure of primary normal human epidermal keratinocytes (NHEK) and primary normal human dermal fibroblasts (NHDF) to SM significantly impaired hypoxia-induced HIF-1α stabilization and target gene expression in these cells. Addition of a selective inhibitor of the oxygen-sensitive prolyl hydroxylase domain-containing protein 2 (PHD-2), IOX2, fully recovered HIF-1α stability, nuclear translocation, and target gene expression in NHEK and NHDF. Moreover, functional studies using a scratch wound assay demonstrated that the application of IOX2 efficiently counteracted SM-mediated deficiencies in monolayer regeneration under hypoxic conditions in NHEK and NHDF. Our findings describe a pathomechanism by which SM negatively affects hypoxia-stimulated HIF-1α signaling in keratinocytes and fibroblasts and thus possibly contributes to delayed wound healing in SM-injured patients that could be treated with PHD-2 inhibitors.





Similar content being viewed by others
Abbreviations
- BNIP3:
-
BCL2/adenovirus E1B 19-kDa protein-interacting protein 3
- HIF-1α:
-
Hypoxia-inducible factor 1 alpha
- MMP-2:
-
Matrix metalloproteinase 2
- MMP-9:
-
Matrix metalloproteinase 9
- NHDF:
-
Normal human dermal fibroblasts
- NHEK:
-
Normal human epidermal keratinocytes
- PHD-2:
-
Prolyl hydroxylase domain-containing protein 2
- VEGF-A:
-
Vascular endothelial growth factor a
- SM:
-
Sulfur mustard
References
Balali-Mood M, Hefazi M, Mahmoudi M et al (2005) Long-term complications of sulphur mustard poisoning in severely intoxicated Iranian veterans. Fundam Clin Pharmacol 19(6):713–721
Bellot G, Garcia-Medina R, Gounon P et al (2009) Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol 29(10):2570–2581
Benita Y, Kikuchi H, Smith AD, Zhang MQ, Chung DC, Xavier RJ (2009) An integrative genomics approach identifies hypoxia inducible factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res 37(14):4587–4602
Bruick RK (2000) Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci USA 97(16):9082–9087
Buth H, Wolters B, Hartwig B et al (2004) HaCaT keratinocytes secrete lysosomal cysteine proteinases during migration. Eur J Cell Biol 83(11–12):781–795
Catrina SB, Okamoto K, Pereira T, Brismar K, Poellinger L (2004) Hyperglycemia regulates hypoxia-inducible factor-1alpha protein stability and function. Diabetes 53(12):3226–3232
Chowdhury R, Candela-Lena JI, Chan MC et al (2013) Selective small molecule probes for the hypoxia inducible factor (HIF) prolyl hydroxylases. ACS Chem Biol 8(7):1488–1496
Dehne N, Hintereder G, Brune B (2010) High glucose concentrations attenuate hypoxia-inducible factor-1alpha expression and signaling in non-tumor cells. Exp Cell Res 316(7):1179–1189
Detmar M, Brown LF, Berse B et al (1997) Hypoxia regulates the expression of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) and its receptors in human skin. J Invest Dermatol 108(3):263–268
Egea V, von Baumgarten L, Schichor C et al (2011) TNF-alpha respecifies human mesenchymal stem cells to a neural fate and promotes migration toward experimental glioma. Cell Death Differ 18(5):853–863
Fraisl P, Aragones J, Carmeliet P (2009) Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov 8(2):139–152
Graham JS, Chilcott RP, Rice P, Milner SM, Hurst CG, Maliner BI (2005) Wound healing of cutaneous sulfur mustard injuries: strategies for the development of improved therapies. J Burns Wounds 4:e1
Graham JS, Stevenson RS, Mitcheltree LW et al (2009) Medical management of cutaneous sulfur mustard injuries. Toxicology 263(1):47–58
Kallio PJ, Wilson WJ, O’Brien S, Makino Y, Poellinger L (1999) Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. J Biol Chem 274(10):6519–6525
Kalucka J, Ettinger A, Franke K et al (2013) Loss of epithelial hypoxia-inducible factor prolyl hydroxylase 2 accelerates skin wound healing in mice. Mol Cell Biol 33(17):3426–3438
Kehe K, Szinicz L (2005) Medical aspects of sulphur mustard poisoning. Toxicology 214(3):198–209
Mace KA, Yu DH, Paydar KZ, Boudreau N, Young DM (2007) Sustained expression of Hif-1alpha in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regen 15(5):636–645
Martens ME, Smith WJ (2008) The role of NAD+ depletion in the mechanism of sulfur mustard-induced metabolic injury. Cutan Ocul Toxicol 27(1):41–53
Martins VL, Caley M, O’Toole EA (2013) Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res 351(2):255–268
Moriyama M, Moriyama H, Uda J, Matsuyama A, Osawa M, Hayakawa T (2014) BNIP3 plays crucial roles in the differentiation and maintenance of epidermal keratinocytes. J Invest Dermatol 134(6):1627–1635
Murray JK, Balan C, Allgeier AM et al (2010) Dipeptidyl-quinolone derivatives inhibit hypoxia inducible factor-1alpha prolyl hydroxylases-1, -2, and -3 with altered selectivity. J Comb Chem 12(5):676–686
O’Toole EA, Marinkovich MP, Peavey CL et al (1997) Hypoxia increases human keratinocyte motility on connective tissue. J Clin Invest 100(11):2881–2891
Polzer H, Haasters F, Prall WC et al (2010) Quantification of fluorescence intensity of labeled human mesenchymal stem cells and cell counting of unlabeled cells in phase-contrast imaging: an open-source-based algorithm. Tissue Eng Part C Methods 16(6):1277–1285
Popp T, Egea V, Kehe K et al (2011) Sulfur mustard induces differentiation in human primary keratinocytes: opposite roles of p38 and ERK1/2 MAPK. Toxicol Lett 204(1):43–51
Rezvani HR, Ali N, Serrano-Sanchez M et al (2011) Loss of epidermal hypoxia-inducible factor-1alpha accelerates epidermal aging and affects re-epithelialization in human and mouse. J Cell Sci 124(Pt 24):4172–4183
Ridgway PF, Ziprin P, Peck DH, Darzi AW (2005) Hypoxia increases reepithelialization via an alphavbeta6-dependent pathway. Wound Repair Regen 13(2):158–164
Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P (2007) MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood 109(9):4055–4063
Ries C, Popp T, Egea V, Kehe K, Jochum M (2009) Matrix metalloproteinase-9 expression and release from skin fibroblasts interacting with keratinocytes: upregulation in response to sulphur mustard. Toxicology 263(1):26–31
Rossiter H, Barresi C, Pammer J et al (2004) Loss of vascular endothelial growth factor a activity in murine epidermal keratinocytes delays wound healing and inhibits tumor formation. Cancer Res 64(10):3508–3516
Rowell M, Kehe K, Balszuweit F, Thiermann H (2009) The chronic effects of sulfur mustard exposure. Toxicology 263(1):9–11
Ruthenborg RJ, Ban JJ, Wazir A, Takeda N, Kim JW (2014) Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1. Mol Cells 37(9):637–643
Saladi RN, Smith E, Persaud AN (2006) Mustard: a potential agent of chemical warfare and terrorism. Clin Exp Dermatol 31(1):1–5
Salo T, Lyons JG, Rahemtulla F, Birkedal Hansen H, Larjava H (1991) Transforming growth factor-beta 1 up-regulates type IV collagenase expression in cultured human keratinocytes. J Biol Chem 266:11436–11441
Sanderson H, Fauser P, Thomsen M, Sorensen PB (2009) Human health risk screening due to consumption of fish contaminated with chemical warfare agents in the Baltic Sea. J Hazard Mater 162(1):416–422
Scholz CC, Taylor CT (2013) Targeting the HIF pathway in inflammation and immunity. Curr Opin Pharmacol 13(4):646–653
Semenza GL (2014) Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol 9:47–71
Sen CK (2009) Wound healing essentials: let there be oxygen. Wound Repair Regen 17(1):1–18
Sugendran K, Jeevaratnam K, Husain K, Singh R, Srivastava DK (1992) Effects of topically applied sulphur mustard on tissue glycogen, blood glucose, lactate and pyruvate in mice. Indian J Physiol Pharmacol 36(3):219–221
Tian YM, Yeoh KK, Lee MK et al (2011) Differential sensitivity of hypoxia inducible factor hydroxylation sites to hypoxia and hydroxylase inhibitors. J Biol Chem 286(15):13041–13051
Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF (2007) BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol 27(17):6229–6242
Zhang X, Yan X, Cheng L et al (2013) Wound healing improvement with PHD-2 silenced fibroblasts in diabetic mice. PLoS One 8(12):e84548
Zimmermann AS, Morrison SD, Hu MS et al (2014) Epidermal or dermal specific knockout of PHD-2 enhances wound healing and minimizes ischemic injury. PLoS One 9(4):e93373
Acknowledgments
This work was funded by grants from the Institute of Cardiovascular Prevention, Ludwig-Maximilians-University of Munich and by a contract of the German Armed Forces (M/SABX/BA003). We especially want to thank Thomas Pitsch for valuable technical assistance in C.R.’s laboratory, Christian Mahl for help with the analysis of the scratch wound assay, Donato Santovito for support in statistical analysis, and Mina Zarrabi as well as Ram Prasad for conducting the intoxication of cells at the Bundeswehr Institute of Pharmacology and Toxicology.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deppe, J., Popp, T., Egea, V. et al. Impairment of hypoxia-induced HIF-1α signaling in keratinocytes and fibroblasts by sulfur mustard is counteracted by a selective PHD-2 inhibitor. Arch Toxicol 90, 1141–1150 (2016). https://doi.org/10.1007/s00204-015-1549-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1549-y