Abstract
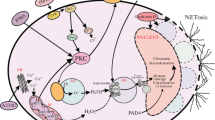
Humans are exposed to different mercurial compounds from various sources, most frequently from dental fillings, preservatives in vaccines, or consumption of fish. Among other toxic effects, these substances interact with the immune system. In high doses, mercurials are immunosuppressive. However, lower doses of some mercurials stimulate the immune system, inducing different forms of autoimmunity, autoantibodies, and glomerulonephritis in rodents. Furthermore, some studies suggest a connection between mercury exposure and the occurrence of autoantibodies against nuclear components and granulocyte cytoplasmic proteins in humans. Still, the underlying mechanisms need to be clarified. The present study investigates the formation of neutrophil extracellular traps (NETs) in response to thimerosal and its metabolites ethyl mercury (EtHg), thiosalicylic acid, and mercuric ions (Hg2+). Only EtHg and Hg2+ triggered NETosis. It was independent of PKC, ERK1/2, p38, and zinc signals and not affected by the NADPH oxidase inhibitor DPI. Instead, EtHg and Hg2+ triggered NADPH oxidase-independent production of ROS, which are likely to be involved in mercurial-induced NET formation. This finding might help understanding the autoimmune potential of mercurial compounds. Some diseases, to which a connection with mercurials has been shown, such as Wegener’s granulomatosis and systemic lupus erythematosus, are characterized by high prevalence of autoantibodies against neutrophil-specific auto-antigens. Externalization in the form of NETs may be a source for exposure to these self-antigens. In genetically susceptible individuals, this could be one step in the series of events leading to autoimmunity.






Similar content being viewed by others
References
Albert D, Clarkin C, Komoroski J, Brensinger CM, Berlin JA (2004) Wegener’s granulomatosis: possible role of environmental agents in its pathogenesis. Arthritis Rheum 51:656–664
Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A (2012) Neutrophil function: from mechanisms to disease. Annu Rev Immunol 30:459–489
Arnett FC, Fritzler MJ, Ahn C, Holian A (2000) Urinary mercury levels in patients with autoantibodies to U3-RNP (fibrillarin). J Rheumatol 27:405–410
Aschner M, Ceccatelli S (2010) Are neuropathological conditions relevant to ethylmercury exposure? Neurotox Res 18:59–68
Barnes DM, Kircher EA (2005) Effects of mercuric chloride on glucose transport in 3T3-L1 adipocytes. Toxicol In Vitro 19:207–214
Branzk N, Papayannopoulos V (2013) Molecular mechanisms regulating NETosis in infection and disease. Semin Immunopathol 35:513–530
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A (2004) Neutrophil extracellular traps kill bacteria. Science 303:1532–1535
Bruns S, Kniemeyer O, Hasenberg M, Aimanianda V, Nietzsche S, Thywissen A, Jeron A, Latgé JP, Brakhage AA, Gunzer M (2010) Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog 6:e1000873
Clarkson TW, Vyas JB, Ballatori N (2007) Mechanisms of mercury disposition in the body. Am J Ind Med 50:757–764
Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Dooley MA (2004) Occupational risk factors for the development of systemic lupus erythematosus. J Rheumatol 31:1928–1933
Darrah E, Andrade F (2012) NETs: The missing link between cell death and systemic autoimmune diseases? Front Immunol 3:428
Elferink JG (1999) Thimerosal: a versatile sulfhydryl reagent, calcium mobilizer, and cell function-modulating agent. Gen Pharmacol 33:1–6
Elferink JG, de Koster BM (1998) The effect of thimerosal on neutrophil migration: a comparison with the effect on calcium mobilization and CD11b expression. Biochem Pharmacol 55:305–312
Esnault VL, Mathieson PW, Thiru S, Oliveira DB, Martin-Lockwood C (1992) Autoantibodies to myeloperoxidase in brown Norway rats treated with mercuric chloride. Lab Invest 67:114–120
Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A (2007) Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176:231–241
Gray RD, Lucas CD, Mackellar A, Li F, Hiersemenzel K, Haslett C, Davidson DJ, Rossi AG (2013) Activation of conventional protein kinase C (PKC) is critical in the generation of human neutrophil extracellular traps. J Inflamm (Lond) 10:12
Haase H, Hebel S, Engelhardt G, Rink L (2006) Flow cytometric measurement of labile zinc in peripheral blood mononuclear cells. Anal Biochem 352:222–230
Haase H, Hebel S, Engelhardt G, Rink L (2009) Zinc ions cause the thimerosal-induced signal of fluorescent calcium probes in lymphocytes. Cell Calcium 45:185–191
Haase H, Engelhardt G, Hebel S, Rink L (2011) Mercuric ions inhibit mitogen-activated protein kinase dephosphorylation by inducing reactive oxygen species. Toxicol Appl Pharmacol 250:78–86
Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, Waldmann H (2011) Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol 7:75–77
Hasan R, Rink L, Haase H (2013) Zinc signals in neutrophil granulocytes are required for the formation of neutrophil extracellular traps. Innate Immun 19:253–264
Haurand M, Flohe L (1989) Leukotriene formation by human polymorphonuclear leukocytes from endogenous arachidonate. Physiological triggers and modulation by prostanoids. Biochem Pharmacol 38:2129–2137
Havarinasab S, Hultman P (2005) Organic mercury compounds and autoimmunity. Autoimmun Rev 4:270–275
Havarinasab S, Haggqvist B, Bjorn E, Pollard KM, Hultman P (2005) Immunosuppressive and autoimmune effects of thimerosal in mice. Toxicol Appl Pharmacol 204:109–121
Jorissen A, Plum LM, Rink L, Haase H (2013) Impact of lead and mercuric ions on the interleukin-2-dependent proliferation and survival of T cells. Arch Toxicol 87:249–258
Kehl-Fie TE, Skaar EP (2010) Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol 14:218–224
Keshari RS, Verma A, Barthwal MK, Dikshit M (2013) Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J Cell Biochem 114:532–540
Kim SH, Sharma RP (2004) Mercury-induced apoptosis and necrosis in murine macrophages: role of calcium-induced reactive oxygen species and p38 mitogen-activated protein kinase signaling. Toxicol Appl Pharmacol 196:47–57
Lauwerys R, Bernard A, Roels H, Buchet JP, Gennart JP, Mahieu P, Foidart JM (1983) Anti-laminin antibodies in workers exposed to mercury vapour. Toxicol Lett 17:113–116
Lund BO, Miller DM, Woods JS (1991) Mercury-induced H2O2 production and lipid peroxidation in vitro in rat kidney mitochondria. Biochem Pharmacol 42(Suppl):S181–S187
Mattingly RR, Felczak A, Chen CC, McCabe MJ Jr, Rosenspire AJ (2001) Low concentrations of inorganic mercury inhibit Ras activation during T cell receptor-mediated signal transduction. Toxicol Appl Pharmacol 176:162–168
Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC (2012) Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukoc Biol 92:841–849
Schiraldi M, Monestier M (2009) How can a chemical element elicit complex immunopathology? Lessons from mercury-induced autoimmunity. Trends Immunol 30:502–509
Shenker BJ, Guo TL, Insug O, Shapiro IM (1999) Induction of apoptosis in human T-cells by methyl mercury: temporal relationship between mitochondrial dysfunction and loss of reductive reserve. Toxicol Appl Pharmacol 157:23–35
Silbergeld EK, Silva IA, Nyland JF (2005) Mercury and autoimmunity: implications for occupational and environmental health. Toxicol Appl Pharmacol 207:282–292
Turney KD, Parrish AR, Orozco J, Gandolfi AJ (1999) Selective activation in the MAPK pathway by Hg(II) in precision-cut rabbit renal cortical slices. Toxicol Appl Pharmacol 160:262–270
Uchida T, Naito S, Kato H, Hatano I, Harashima A, Terada Y, Ohkawa T, Chino F, Eto K (1994) Thimerosal induces toxic reaction in non-sensitized animals. Int Arch Allergy Immunol 104:296–301
Vas J, Monestier M (2008) Immunology of mercury. Ann N Y Acad Sci 1143:240–267
Yipp BG, Kubes P (2013) NETosis: How vital is it? Blood 122:2784–2794
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haase, H., Hebel, S., Engelhardt, G. et al. Ethylmercury and Hg2+ induce the formation of neutrophil extracellular traps (NETs) by human neutrophil granulocytes. Arch Toxicol 90, 543–550 (2016). https://doi.org/10.1007/s00204-015-1484-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1484-y