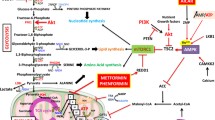
Abstract
Significant efforts have been made for the development of new anticancer drugs (protein kinase or proteasome inhibitors, monoclonal humanized antibodies) with presumably low or negligible side effects and high specificity. However, an in-depth analysis of the side effects of several currently used canonical (platin-based drugs, taxanes, anthracyclines, etoposides, antimetabolites) and new generation anticancer drugs as the first line of clinical treatment reveals significant perturbation of glycolysis and oxidative phosphorylation. Canonical and new generation drug side effects include decreased (1) intracellular ATP levels, (2) glycolytic/mitochondrial enzyme/transporter activities and/or (3) mitochondrial electrical membrane potentials. Furthermore, the anti-proliferative effects of these drugs are markedly attenuated in tumor rho (0) cells, in which functional mitochondria are absent; in addition, several anticancer drugs directly interact with isolated mitochondria affecting their functions. Therefore, several anticancer drugs also target the energy metabolism, and hence, the documented inhibitory effect of anticancer drugs on cancer growth should also be linked to the blocking of ATP supply pathways. These often overlooked effects of canonical and new generation anticancer drugs emphasize the role of energy metabolism in maintaining cancer cells viable and its targeting as a complementary and successful strategy for cancer treatment.




Similar content being viewed by others
Abbreviations
- ETC:
-
Electron transport chain
- OxPhos:
-
Oxidative phosphorylation
- 2-OGDH:
-
2-Oxoglutarate dehydrogenase complex
References
Abdel-aleem S, el-Merzabani MM, Sayed-Ahmed M, Taylor DA, Lowe JE (1997) Acute and chronic effects of adriamycin on fatty acid oxidation in isolated cardiac myocytes. J Mol Cell Cardiol 29:789–797. doi:10.1006/jmcc.1996.0323
Aggarwal SK (1993) A histochemical approach to the mechanism of action of cisplatin and its analogues. J Histochem Cytochem 41:1053–1073. doi:10.1177/41.7.8515048
Alas S, Ng CP, Bonavida B (2002) Rituximab modifies the cisplatin-mitochondrial signaling pathway, resulting in apoptosis in cisplatin-resistant non-Hodgkin’s lymphoma. Clin Cancer Res 8:836–845. http://clincancerres.aacrjournals.org/content/8/3/836
Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, Tempel W, Dimov S, Shen M, Jha A et al (2012) Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol 8:839–847. doi:10.1038/nchembio.1060
André N, Braguer D, Brasseur G, Gonçalves A, Lemesle-Meunier D, Guise S, Jordan MA, Briand C (2000) Paclitaxel induces release of cytochrome c from mitochondria isolated from human neuroblastoma cells. Cancer Res 60:5349–5353. http://cancerres.aacrjournals.org/content/60/19/5349
Angelucci A, Valentini A, Millimaggi D, Gravina GL, Miano R, Dolo V, Vicentini C, Bologna M, Federici G, Bernardini S (2006) Valproic acid induces apoptosis in prostate carcinoma cell lines by activation of multiple death pathways. Anticancer Drugs 17:1141–1150. doi:10.1097/01.cad.0000236302.89843.fc
Arce C, Pérez-Plasencia C, González-Fierro A, de la Cruz-Hernández E, Revilla-Vázquez A, Chávez-Blanco A, Trejo-Becerril C, Pérez-Cárdenas E, Taja-Chayeb L, Bargallo E et al (2006) A proof-of-principle study of epigenetic therapy added to neoadjuvant doxorubicin cyclophosphamide for locally advanced breast cancer. PLoS One 1:e98. doi:10.1371/journal.pone.0000098
Ardizzoni A, Addamo GF, Baldini E, Borghini U, Portalone L, De Marinis F, Lionetto R, Conte PF, Bruzzi P, Pennucci MC et al (1995) Mitomycin-ifosfamide-cisplatinum (MIP) vs MIP-interferon vs cisplatinum-carboplatin in metastatic non-small-cell lung cancer: a FONICAP randomised phase II study. Ital Lung Cancer Task Force Br J Cancer 71:115–119. doi:10.1038/bjc.1995.23
Arora A, Scholar EM (2005) Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther 315:971–979. doi:10.1124/jpet.105.084145
Bai S, Nasse MW, Wang SH, Datta J, Kutay H, Yadav A, Nuovo G, Kumar P, Ghoshal K (2009) MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem 284(46):32015–32027. doi:10.1074/jbc.M109.016774
Baldo BA (2013) Adverse events to monoclonal antibodies used for cancer therapy: focus on hypersensitivity responses. Oncoimmunology 2:e26333. doi:10.4161/onci.26333
Barbone D, Cheung P, Battula S, Busacca S, Gray SG, Longley DB, Bueno R, Sugarbaker DJ, Fennell DA, Broaddus VC (2012) Vorinostat eliminates multicellular resistance of mesothelioma 3D spheroids via restoration of Noxa expression. PLoS One 7:e52753. doi:10.1371/journal.pone.0052753
Barnes K, McIntosh E, Whetton AD, Daley GQ, Bentley J, Baldwin SA (2005) Chronic myeloid leukaemia: an investigation into the role of Bcr-Abl-induced abnormalities in glucose transport regulation. Oncogene 24:3257–3267. doi:10.1038/sj.onc.1208461
Bilbro J, Mart M, Kyprianou M (2013) Therapeutic value of quinazoline-based compounds in prostate cancer. Anticancer Res 33:4695–4700
Blackburn C, Gigstad KM, Hales P, Garcia K, Jones M, Bruzzese FJ, Barrett C, Liu JX, Soucy TA, Sappal DS et al (2010) Characterization of a new series of non-covalent proteasome inhibitors with exquisite potency and selectivity for the 20S beta5-subunit. Biochem J 430:461–476. doi:10.1042/BJ20100383
Blaheta RA, Michaelis M, Driever PH, Cinatl J Jr (2005) Evolving anticancer drug valproic acid: insights into the mechanism and clinical studies. Med Res Rev 25:383–397. doi:10.1002/med.20027
Blandino G, Valerio M, Cioce M, Mori F, Casadei L, Pulito C, Sacconi A, Biagioni F, Cortese G, Galanti S et al (2012) Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat Commun 29:865. doi:10.1038/ncomms1859
Boren J, Cascante M, Marin S, Comín-Anduix B, Centelles JJ, Lim S, Bassilian S, Ahmed S, Lee WN, Boros LG (2001) Gleevec (STI571) influences metabolic enzyme activities and glucose carbon flow toward nucleic acid and fatty acid synthesis in myeloid tumor cells. J Biol Chem 276:37747–37753. doi:10.1074/jbc.M105796200
Boros LG, Lee WN, Cascante M (2002) Imatinib and chronic-phase leukemias. N Engl J Med 347:67–68. doi:10.1056/NEJM200207043470116
Braiteh F, Soriano AO, Garcia-Manero G, Hong D, Johnson MM, Silva LDP, Yang H, Alexander S, Wolff J, Kurzrock R (2008) Phase I study of epigenetic modulation with 5-azacytidine and valproic acid in patients with advanced cancers. Clin Cancer Res 14:6296–62301. doi:10.1158/1078-0432.CCR-08-1247
Brower JV, Lim CH, Han C, Hankowski KE, Hamazaki T, Terada N (2009) Differential CpG island methylation of murine adenine nucleotide translocase genes. Biochim Biophys Acta 1789:198–203. doi:10.1016/j.bbagrm.2008.12.005
Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhäusl W, Fürnsinn C (2004) Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes 53:1052–1059. doi:10.2337/diabetes.53.4.1052
Bugger H, Guzman C, Zechner C, Palmeri M, Russell KS, Russell RR 3rd (2011) Uncoupling protein downregulation in doxorubicin-induced heart failure improves mitochondrial coupling but increases reactive oxygen species generation. Cancer Chemother Pharmacol 67:1381–1388. doi:10.1007/s00280-010-1441-7
Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB (2007) Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res 67:6745–6752. doi:10.1158/0008-5472.CAN-06-4447
Byczkowski JZ, Zychlinski L, Porter CW (1982) Potentiation of the antimitochondrial and antiproliferative effects of bis(guanylhydrazones) by phenethylbiguanide. Cancer Res 42:3592–3595. http://cancerres.aacrjournals.org/content/42/9/3592
Cairns RA, Papandreou I, Sutphin PD, Denko NC (2007) Metabolic targeting of hypoxia and HIF1 in solid tumors can enhance cytotoxic chemotherapy. Proc Natl Acad Sci USA 104:9445–9450. doi:10.1073/pnas.0611662104
Candi E, Agostini M, Melino M, Bernassola F (2014) How the TP53 family proteins TP63 and TP73 contribute to tumorigenesis: regulators and effectors. Hum Mutat doi:10.1002/humu.22523
Carvalho C, Correia S, Santos MS, Seiça R, Oliveira CR, Moreira PI (2008) Metformin promotes isolated rat liver mitochondria impairment. Mol Cell Biochem 308:75–83. doi:10.1007/s11010-007-9614-3
Cecconi D, Donadelli M, Dalla Pozza E, Rinalducci S, Zolla L, Scupoli MT, Righetti PG, Scarpa A, Palmieri M (2009) Synergistic effect of trichostatin A and 5-aza-2′-deoxycytidine on growth inhibition of pancreatic endocrine tumour cell lines: a proteomic study. Proteomics 9:1952–1966. doi:10.1002/pmic.200701089
Chan A (2007) A review of the use of trastuzumab (Herceptin) plus vinorelbine in metastatic breast cancer. Ann Oncol 18:1152–1158. doi:10.1093/annonc/mdl476
Chan CL, Wu Z, Ciardelli T, Eastman A, Bresnick E (1993) Kinetic and DNA-binding properties of recombinant human O 6-methylguanine-DNA methyltransferase. Arch Biochem Biophys 300:193–200. doi:10.1006/abbi.1993.1027
Chen Z, Li Y, Zhang H, Huang P, Luthra R (2010) Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene 29:4362–4368. doi:10.1038/onc.2010.193
Cherepanova NA, Minero AS, Rakhimova AR, Gromova ES (2011) Mechanism of CpG DNA methyltransferases M. SssI and Dnmt3a studied by DNA containing 2-aminopurine. Nucleosides, Nucleotides Nucleic Acids 30:619–631. doi:10.1080/15257770.2011.583973
Chesney J, Telang S (2013) Regulation of glycolytic and mitochondrial metabolism by ras. Curr Pharm Biotechnol 14:251–260. doi:10.2174/1389201011314030002
Cheung KJ, Gabrielson E, Werb Z, Ewald A (2013) Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 155:1639–1651. doi:10.1016/j.cell.2013.11.029
Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J et al (2007) Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 370:2011–2019. doi:10.1016/S0140-6736(07)61865-0
Clark SJ, Melki J (2002) DNA methylation and gene silencing in cancer: which is the guilty party? Oncogene 21:5380–5387. doi:10.1038/sj.onc.1205598
Coronel J, Cetina L, Pacheco I, Trejo-Becerril C, González-Fierro A, de la Cruz-Hernandez E, Perez-Cardenas E, Taja-Chayeb L, Arias-Bofill D, Candelaria M et al (2011) A double-blind, placebo-controlled, randomized phase III trial of chemotherapy plus epigenetic therapy with hydralazine valproate for advanced cervical cancer. Preliminary results. Med Oncol 28:S540–S546. doi:10.1007/s12032-010-9700-3
Cuéllar A, Escamilla E, Ramírez J, Chávez E (1984) Adriamycin as an inhibitor of 11 beta-hydroxylase activity in adrenal cortex mitochondria. Arch Biochem Biophys 235:538–543. doi:10.1016/0003-9861(84)90227-3
Cummings J, Spanswick VJ, Smyth JF (1995) Re-evaluation of the molecular pharmacology of mitomycin C. Eur J Cancer 31A:1928–1933. doi:10.1016/0959-8049(95)00364-9
Custódio JB, Cardoso CM, Almeida LM (2002) Thiol protecting agents and antioxidants inhibit the mitochondrial permeability transition promoted by etoposide: implications in the prevention of etoposide-induced apoptosis. Chem Biol Interact 140:169–184. doi:10.1016/S0009-2797(2)00020-0
Dang CV, Le A, Gao P (2009) MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res 15:6479–6483
Dao T, Yan S, Veomett N, Pankov D, Zhou L, Korontsvit T, Scott A, Whitten J, Maslak P, Casey E, et al (2013) Targeting the intracellular WT1 oncogene product with a therapeutic human antibody. Sci Transl Med 5:176ra33. doi:10.1126/scitranslmed.3005661
den Hollander P, Savage MI, Brown PH (2013) Targeted Therapy for Breast Cancer Prevention. Front Oncol 3:250. doi:10.1158/1078-0432.CCR-09-0889
Ding Y, Liu Z, Desai S, Zhao Y, Liu H, Pannell LK, Yi H, Wright ER, Owen LB, Dean-Colomb W et al (2012) Receptor tyrosine kinase ErbB2 translocates into mitochondria and regulates cellular metabolism. Nat Commun 3:1271. doi:10.1038/ncomms2236
Diotte NM, Xiong Y, Gao J, Chua BH, Ho YS (2009) Attenuation of doxorubicin-induced cardiac injury by mitochondrial glutaredoxin 2. Biochim Biophys Acta 1793:427–438. doi:10.1016/j.bbamcr.2008.10.014
Drahota Z, Palenickova E, Endlicher R, Milerova M, Brejchova J, Vosahlikova M, Svoboda P, Kazdova L, Kalous M, Cervinkova Z, Cahova M (2013) Biguanides inhibit complex I, II and IV of rat liver mitochondria and modify their functional properties. Physiol Res 63:1–11
Dykens JA, Jamieson J, Marroquin L, Nadanaciva S, Billis PA, Will Y (2008) Biguanide-induced mitochondrial dysfunction yields increased lactate production and cytotoxicity of aerobically-poised HepG2 cells and human hepatocytes in vitro. Toxicol Appl Pharmacol 233:203–210. doi:10.1016/j.taap.2008.08.013
Eeva J, Nuutinen U, Ropponen A, Mättö M, Eray M, Pellinen R, Wahlfors J, Pelkonen J (2009) The involvement of mitochondria and the caspase-9 activation pathway in rituximab-induced apoptosis in FL cells. Apoptosis 14:687–698. doi:10.1007/s10495-009-0337-7
El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 275:223–228. http://www.jbc.org/content/275/1/223
Esteller M (2007) Epigenetics provides a new generation of oncogenes and tumour-suppressor genes. Br J Cancer 96:R26–R30. doi:10.1038/sj.bjc.6602918
Fabian MA, Biggs WH, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M et al (2005) A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol 23:329–336. doi:10.1038/nbt1068
Fantin VR, Berardi MJ, Babbe H, Michelman MV, Manning CM, Leder P (2005) A bifunctional targeted peptide that blocks HER-2 tyrosine kinase and disables mitochondrial function in HER-2-positive carcinoma cells. Cancer Res 65:6891–6900. http://cancerres.aacrjournals.org/content/65/15/6891
Faure-Vigny H, Heddi A, Giraud S, Chautard D, Stepien G (1996) Expression of oxidative phosphorylation genes in renal tumors and tumoral cell lines. Mol Carcinog 16:165–172. doi:10.1002/(SICI)1098-2744(199607
Ferlini C, Raspaglio G, Mozzetti S, Distefano M, Filippetti F, Martinelli E, Ferrandina G, Gallo D, Ranelletti FO, Scambia G (2003) Bcl-2 down-regulation is a novel mechanism of paclitaxel resistance. Mol Pharmacol 64:51–58. doi:10.1124/mol.64.1.51
Fischer Y, Thomas J, Rösen P, Kammermeier H (1995) Action of metformin on glucose transport and glucose transporter GLUT1 and GLUT4 in heart muscle cells from healthy and diabetic rats. Endocrinology 136:412–420. doi:10.1210/endo.136.2.7835271
Fiume L, Manerba M, Vettraino M, Di Stefano G (2011) Effect of sorafenib on the energy metabolism of hepatocellular carcinoma cells. Eur J Pharmacol 670:39–43. doi:10.1016/j.ejphar.2011.08.038
Floridi A, D’Atri S, Bellocci M, Marcante ML, Paggi MG, Silvestrini B, Caputo A, De Martino C (1984) The effect of gossypol and Lonidamine on electron transport in Ehrlich ascites tumor mitochondria. Exp Mol Pathol 40:246–261. doi:10.1016/0014-4800(84)90081-9
Funasaka T, Hogan V, Raz A (2009) Phosphoglucose isomerase/autocrine motility factor mediates epithelial and mesenchymal phenotype conversions in breast cancer. Cancer Res 69:5349–5356. doi:10.1158/0008-5472.CAN-09-0488
Gallardo-Pérez JC, Rivero-Segura NA, Marín-Hernández A, Moreno-Sánchez R, Rodríguez-Enríques S (2014) GPI/AMF inhibition blocks the metastatic progression of mature multicellular tumor spheroids. Biochim Biophys Acta 1843:1043–1053. doi:10.1016/j.bbamcr.2014.01.013
Gaona-Gaona L, Molina-Jijón E, Tapia E, Zazueta C, Hernández-Pando R, Calderón-Oliver M, Zarco-Márquez G, Pinzón E, Pedraza-Chaverri J (2011) Protective effect of sulforaphane pretreatment against cisplatin-induced liver and mitochondrial oxidant damage in rats. Toxicology 286:20–27. doi:10.1016/j.tox.2011.04.014
Garrido N, Pérez-Martos A, Faro M, Lou-Bonafonte JM, Fernández-Silva P, López-Pérez MJ, Montoya J, Enríquez JA (2008) Cisplatin-mediated impairment of mitochondrial DNA metabolism inversely correlates with glutathione levels. Biochem J 414:93–102. doi:10.1042/BJ20071615
Gettings SD, Reeve JE, King LJ (1988) Possible role of intracellular Ca2+ in the toxicity of phenformin. Biochem Pharmacol 37:281–289. doi:10.1016/0006-2952(88)90730-7
Giacobbe A, Bongiorno-Borbone L, Bernassola F, Terrinoni A, Markert EK, Levine AJ, Feng Z, Agostini M, Zolla L, Agro AF, Notterman DA, Melino G, Peschiaroli A (2013) p63 regulates glutaminase 2 expression. Cell Cycle 12:1395–1405. doi:10.4161/cc.24478
Gillies RJ, Robey I, Gatenby RA (2008) Causes and consequences of increased glucose metabolism of cancers. J Nucl Med 49:24S–42S. doi:10.2967/jnumed.107.047258
Gottschalk S, Anderson N, Hainz C, Eckhardt SG, Serkova NJ (2004) Imatinib (STI571)-mediated changes in glucose metabolism in human leukemia BCR-ABL-positive cells. Clin Cancer Res 10:6661–6668. doi:10.1158/1078-0432.CCR-04-0039
Gowher H, Jeltsch A (2004) Mechanism of inhibition of DNA methyltransferases by cytidine analogs in cancer therapy. Cancer Biol Ther 3:1062–1068. doi:10.4161/cbt.3.11.1308
Grazette LP, Boecker W, Matsui T, Semigran M, Force TL, Hajjar RJ, Rosenzweig A (2004) Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: implications for herceptin-induced cardiomyopathy. J Am Coll Cardiol 44:2231–2238. doi:10.1016/j.jacc.2004.08.066
Guerrero-Beltrán CE, Calderón-Oliver M, Martínez-Abundis E, Tapia E, Zarco-Márquez G, Zazueta C, Pedraza-Chaverri J (2010) Protective effect of sulforaphane against cisplatin-induced mitochondrial alterations and impairment in the activity of NAD(P)H: quinone oxidoreductase 1 and γ glutamyl cysteine ligase: studies in mitochondria isolated from rat kidney and in LLC-PK1 cells. Toxicol Lett 199:80–92. doi:10.1016/j.toxlet.2010.08.009
Guigas B, Detaille D, Chauvin C, Batandier C, De Oliveira F, Fontaine E, Leverve X (2004) Metformin inhibits mitochondrial permeability transition and cell death: a pharmacological in vitro study. Biochem J 382:877–884. doi:10.1042/BJ20040885
Haas R, Stumpf DA, Parks JK, Eguren L (1981) Inhibitory effects of sodium valproate on oxidative phosphorylation. Neurology 31:1473–1476. doi:10.1212/WNL.31.11.1473
Hamann A, Benecke H, Greten H, Hatthaei S (1993) Metformin increases glucose transporter protein and gene expression in human fibroblasts. Biochem Biophys Res Commun 196:382–387. doi:10.1006/bbcr.193.2260
Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ (2010) The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov 9:325–338. doi:10.1038/nrd3003
Haynes BP, Dowsett M, Miller WR, Dixon JM, Bhatnagar AS (2003) The pharmacology of letrozole. J Steroid Biochem Mol Biol 87:35–45. doi:10.1016/S0960-0760(03)00384-4
Hegde PS, Rusnak D, Bertiaux M, Alligood K, Strum J, Gagnon R, Gilmer TM (2007) Delineation of molecular mechanisms of sensitivity to lapatinib in breast cancer cell lines using global gene expression profiles. Mol Cancer Ther 6:1629–1640. doi:10.1158/1535-7163.MCT-05-0399
Hernández-Esquivel L, Marín-Hernández A, Pavón N, Carvajal K, Moreno-Sánchez R (2006) Cardiotoxicity of copper-based antineoplastic drugs casiopeinas is related to inhibition of energy metabolism. Toxicol Appl Pharmacol 212:79–88. doi:10.1016/j.taap.2005.06.023
Hickey FB, Cotter TG (2006) Identification of transcriptional targets associated with the expression of p210 Bcr-Abl. Eur J Haematol 76:369–383. doi:10.1111/j.1600-0609.2006.00629.x
Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M et al (2009) Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res 69:4918–4925. doi:10.1158/0008-5472.CAN-08-4806
Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA (2010) Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol 148:3–15. doi:10.1016/j.jbiotec.2010.01.012
Hirschhaeuser F, Sattler UG, Mueller-Klieser W (2011) Lactate: a metabolic keyplayer in cancer. Cancer Res 71:6921–6925. doi:10.1158/0008-5472.CAN-11-1457
Höckel M, Vaupel P (2001) Biological consequences of tumor hypoxia. Semin Oncol 28:36–41. http://www.seminoncol.org/article/S0093-7754(01)90211-8/abstract
Hodges-Gallagher L, Valentine CD, Bader SE, Kushner PJ (2007) Inhibition of histone deacetylase enhances the anti-proliferative action of antiestrogens on breast cancer cells and blocks tamoxifen-induced proliferation of uterine cells. Breast Cancer Res Treat 105:297–309. doi:10.1007/s10549-006-9459-6
Iacobazzi V, Infantino V, Palmieri F (2008) Epigenetic mechanisms and Sp1 regulate mitochondrial citrate carrier gene expression. Biochem Biophys Res Commun 376:15–20. doi:10.1016/j.bbrc.2008.08.015
Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, Merino M, Trepel J, Zbar B, Toro J, Ratcliffe PJ, Linehan WM, Neckers L (2005) HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8:143–153. doi:10.1016/j.ccr.2005.06.017
Kachadourian R, Brechbuhl HM, Ruiz-Azuara L, Gracia-Mora I, Day BJ (2010) Casiopeína IIgly-induced oxidative stress and mitochondrial dysfunction in human lung cancer A549 and H157 cells. Toxicology 268:176–183. doi:10.1016/j.tox.2009.12.010
Keller KE, Doctor ZM, Dwyer ZW, Lee YS (2014) SAICAR induces protein kinase activity of PKM2 that is necessary for sustained proliferative signaling of cancer cells. Mol Cell 53:700–709. doi:10.1016/j.molcel.2014.02.015
Kessler T, Bayer M, Schwöppe C, Liersch R, Mesters RM, Berdel WE (2010) Compounds in clinical phase III and beyond. In: Liersch R, Berdel WE, Kessler T (eds) Angiogenesis inhibition. Springer, London, pp 137–163
Keunen O, Johansson M, Oudin A, Sanzey M, Rahim SA, Fack F, Thorsen F, Taxt T, Bartos M, Jirik R et al (2011) Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci USA 108:3749–3754. doi:10.1073/pnas.1014480108
Keyes SR, Heimbrook DC, Fracasso PM, Rockwell S, Sligar SG, Sartorelli AC (1985) Chemotherapeutic attack of hypoxic tumor cells by the bioreductive alkylating agent mitomycin C. Adv Enzyme Regul 23:291–307. doi:10.1016/0065-2571(85)90053-6
Kibayashi M, Nagao M, Chiba S (1999) Influence of valproic acid on the expression of various acyl-CoA dehydrogenases in rats. Pediatr Int 41:52–60. doi:10.1046/j.1442-200x.1999.01012.x
Kim MJ, Kim DH, Jung WH, Koo JS (2014) Expression of metabolism-related proteins in triple-negative breast cancer. Int J Clin Exp Pathol 7:301–312. www.ijcep.com. ISSN 1936-2625/IJCEP1311042
Kita A, Mitsuoka K, Kaneko N, Nakata M, Yamanaka K, Jitsuoka M, Miyoshi S, Noda A, Mori M, Nakahara T, Sasamata M (2012) Sepantronium bromide (YM155) enhances response of human B-cell non-Hodgkin lymphoma to rituximab. J Pharmacol Exp Ther 343:178–183. doi:10.1124/jpet.112.195925
Klawitter J, Anderson N, Klawitter J, Christians U, Leibfritz D, Eckhardt SG, Serkova NJ (2009) Time-dependent effects of imatinib in human leukaemia cells: a kinetic NMR-profiling study. Br J Cancer 100:923–931. doi:10.1038/sj.bjc.6604946
Kluza J, Jendoubi M, Ballot C, Dammak A, Jonneaux A, Idziorek T, Joha S, Dauphin V, Malet-Martino M et al (2011) Exploiting mitochondrial dysfunction for effective elimination of imatinib-resistant leukemic cells. PLoS One 6:e21924. doi:10.1371/journal.pone.0021924
Koh MY, Spivak-Kroizman TR, Powis G (2010) HIF-1α and cancer therapy. In: Liersch R, Berdel WE, Kessler T (eds) Angiogenesis inhibition. Springer, London, pp 15–34
Kong M, Ba M, Liang H, Ma L, Yu Q, Yu T, Wang Y (2012) 5′-Aza-dC sensitizes paraquat toxic effects on PC12 cell. Neurosci Lett 524:35–39. doi:10.1016/j.neulet.2012.07.001
Ksienski D (2011) Imatinib mesylate: past successes and future challenges in the treatment of gastrointestinal stromal tumors. Clin Med Insights Oncol 5:365–379. doi:10.4137/CMO.S4259
Kunz-Schughart LA (1999) Multicellular tumor spheroids: intermediates between monolayer culture and in vivo tumor. Cell Biol Int 23:157–161. doi:10.1177/1087057104265040
Kunz-Schughart LA, Freyer JP, Hofstaedter F, Ebner R (2004) The use of 3-D cultures for high-throughput screening: the multicellular spheroid model. J Biomol Screen 9:228–273. doi:10.1177/1087057104265040
Lake DE, Hudis C (2002) Aromatase inhibitors in breast cancer: an update. Cancer Control 9:490-498. http://moffitt.org/File%20Library/Main%20Nav/Research%20and%20Clinical%20Trials/Cancer%20Control%20Journal/v9n6/490.pdf
Le SB, Hailer MK, Buhrow S, Wang Q, Flatten K, Pediaditakis P, Bible KC, Lewis LD, Sausville EA, Pang YP, Ames MM et al (2007) Inhibition of mitochondrial respiration as a source of adaphostin-induced reactive oxygen species and cytotoxicity. J Biol Chem 282:8860–8872. doi:10.1074/jbc.M611777200
Lee SJ, Wang JY (2009) Exploiting the promiscuity of imatinib. J Biol. 8:30. doi:10.1186/jbiol134
Lee CS, Park SY, Ko HH, Han ES (2004) Effect of change in cellular GSH levels on mitochondrial damage and cell viability loss due to mitomycin c in small cell lung cancer cells. Biochem Pharmacol 68:1857–1867. doi:10.1016/j.bcp.2004.06010
Li GN, Wang SP, Xue X, Qu XJ, Liu HP (2013) Monoclonal antibody-related drugs for cancer therapy. Drug Discov Ther 7:178–184. doi:10.5582/ddt.2013.v7.5.178
Lin YL, Meng Y, Jiang W, Roux B (2013) Explaining why Gleevec is a specific and potent inhibitor of Abl kinase. Proc Natl Acad Sci USA 110:1664–1669. doi:10.1073/pnas.1214330110
Liu W, Phang JM (2012) Proline dehydrogenase (oxidase), a mitochondrial tumor suppressor, and autophagy under the hypoxia microenvironment. Autophagy 8:1407–1409
Liu FT, Agrawal SG, Gribben JG, Ye H, Du MQ, Newland AC, Jia L (2008) Bortezomib blocks Bax degradation in malignant B cells during treatment with TRAIL. Blood 111:2797–2805
Liu W, Glunde K, Bhujwalla ZM, Raman V, Sharma A, Phang JM (2012) Proline oxidase promotes tumor cell survival in hypoxic tumor microenvironments. Cancer Res 72:3677–3686
Liu AM, Xu Z, Shek FH, Wong KF, Lee NP, Poon RT, Chen J, Luk JM (2014) miR-122 targets pyruvate kinase M2 and affects metabolism of hepatocellular carcinoma. PLoS ONE 9:e86872
Lu H, Li X, Luo Z, Liu J, Fan Z (2013) Cetuximab reverses the Warburg effect by inhibiting HIF-1-regulated LDH-A. Mol Cancer Ther 12:2187–2199. doi:10.1158/0008-5472.CAN-12-0080
Luwor RB, Lu Y, Li X, Mendelsohn J, Fan Z (2005) The antiepidermal growth factor receptor monoclonal antibody cetuximab/C225 reduces hypoxia-inducible factor-1 alpha, leading to transcriptional inhibition of vascular endothelial growth factor expression. Oncogene 24:4433–4441. doi:10.1038/sj.onc.1208625
Ma WW, Jacene H, Song D, Vilardell F, Messersmith WA, Laheru D, Wahl R, Endres C, Jimeno A, Pomper MG, Hidalgo M (2009) [18F]fluorodeoxyglucose positron emission tomography correlates with Akt pathway activity but is not predictive of clinical outcome during mTOR inhibitor therapy. J Clin Oncol 27:2697–2704. doi:10.1200/JCO.2008.18.8383
Mackay A, Urruticoechea A, Dixon JM, Dexter T, Fenwick K, Ashworth A, Drury S, Larionov A, Young O, White S et al (2007) Molecular response to aromatase inhibitor treatment in primary breast cancer. Breast Cancer Res 9:R37. doi:10.1186/bcr1732
Mandujano-Tinoco EA, Gallardo-Pérez JC, Marín-Hernández A, Moreno-Sánchez R, Rodríguez-Enríquez S (2013) Anti-mitochondrial therapy in human breast cáncer multi-cellular spheroids. Biochim Biophys Acta 1833:541–551. doi:10.1016/j.bbamcr.2012.11.013
Marín-Hernández A, Gracia-Mora I, Ruiz-Ramírez L, Moreno-Sánchez R (2003) Toxic effects of copper-based antineoplastic drugs (Casiopeinas) on mitochondrial functions. Biochem Pharmacol 65:1979–1989. doi:10.1016/S0006-2952(03)00212-0
Marín-Hernández A, Rodríguez-Enríquez S, Vital-González PA, Flores-Rodríguez FL, Macías-Silva M, Sosa-Garrocho M, Moreno-Sánchez R (2006) Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J 273:1975–1988. doi:10.1111/j.1742-4658.2006.05214.x
Marín-Hernández A, Gallardo-Pérez JC, Ralph SJ, Rodríguez-Enríquez S, Moreno-Sánchez R (2009) HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini Rev Med Chem 9:1084–1101. doi:10.2174/138955709788922610
Marín-Hernández A, Gallardo-Pérez JC, Rodríguez-Enríquez S, Encalada R, Moreno-Sánchez R, Saavedra E (2011) Modeling cancer glycolysis. Biochim Biophys Acta 1807:755–767. doi:10.1016/j.bbabio.2010.11.006
Marín-Hernández A, Gallardo-Pérez JC, López-Ramírez SY, García-García JD, Rodríguez-Zavala JS, Ruiz-Ramírez L, Gracia-Mora I, Zentella-Dehesa A, Sosa-Garrocho M, Macías-Silva M, Moreno-Sánchez R, Rodríguez-Enríquez S (2012) Casiopeina II-gly and bromo-pyruvate inhibition of tumor hexokinase, glycolysis, and oxidative phosphorylation. Arch Toxicol 86:753–766. doi:10.1007/s00204-012-0809-3
Masdehors P, Merle-Béral H, Maloum K, Omura S, Magdelénat H, Delic J (2000) Deregulation of the ubiquitin system and p53 proteolysis modify the apoptotic response in B-CLL lymphocytes. Blood 96:269–274. http://bloodjournal.hematologylibrary.org/content/96/1/269.long
Mathews EH, Liebenberg L, Pelzer R (2011) High-glycolytic cancers and their interplay with the body’s glucose demand and supply cycle. Med Hypotheses 76:157–165. doi:10.1016/j.mehy.2010.09.006
Mazieres J, Peters S, Lepage B, Cortot AB, Barlesi F, Beau-Faller M, Besse B, Blons H, Mansuet-Lupo A, Urban T et al (2013) Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 31:1997–2003. doi:10.1200/JCO.2012.45.6095
Mazurek S (2011) Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol 43(2011):969–980
Mellor HR, Bell AR, Valentin JP, Roberts RR (2011) Cardiotoxicity associated with targeting kinase pathways in cancer. Toxicol Sci 120:14–32. doi:10.1093/toxsci/kfq378
Mendelsohn J, Baselga J (2000) The EGF receptor family as targets for cancer therapy. Oncogene 19:6550–6565. doi:10.1038/sj.onc.1204082
Meng F, Sun G, Zhong M, Yu Y, Brewer MA (2013) Anticancer efficacy of cisplatin and trichostatin A or 5-aza-2′-deoxycytidine on ovarian cancer. Br J Cancer 108:579–586. doi:10.1038/bjc.2013.10
Menon C, Polin GM, Prabakaran I, Hsi A, Cheung C, Culver JP, Pingpank JF, Sehgal CS, Yodh AG, Buerk DG et al (2003) An integrated approach to measuring tumor oxygen status using human melanoma xenografts as a model. Cancer Res 63:7232–7240. http://cancerres.aacrjournals.org/content/63/21/7232
Miller WR, Larionov A (2011) Molecular effects of oestrogen deprivation in breast cancer. Mol Cell Endocrinol 340:127–136. doi:10.1097/FPC.0b013e32820b853a
Miller WR, Larionov AA, Renshaw L, Anderson TJ, White S, Murray J, Murray E, Hampton G, Walker JR, Ho S et al (2007) Changes in breast cancer transcriptional profiles after treatment with the aromatase inhibitor, letrozole. Pharmacogenet Genomics 17:813–826
Miller WR, Larionov A, Anderson TJ, Evans DB, Dixon JM (2012) Sequential changes in gene expression profiles in breast cancers during treatment with the aromatase inhibitor, letrozole. Pharmacogenomics J 12:10–21. doi:10.1038/tpj.2010.67
Miller MJ, Foy KC, Kaumaya PT (2013) Cancer immunotherapy: present status, future perspective, and a new paradigm of peptide immunotherapeutics. Discov Med 15:166–176. http://www.discoverymedicine.com/Megan-Jo-Miller/2013/03/28/cancer-immunotherapy-present-status-future-perspective-and-a-new-paradigm-of-peptide-immunotherapeutics/
Mironov SL, Ivannikov MV, Johansson M (2005) [Ca2+]i signaling between mitochondria and endoplasmic reticulum in neurons is regulated by microtubules. From mitochondrial permeability transition pore to Ca2+-induced Ca2+ release. J Biol Chem 280:715–721. doi:10.1074/jbc.M409819200
Montopoli M, Bellanda M, Lonardoni F, Ragazzi E, Dorigo P, Froldi G, Mammi S, Caparrotta L (2011) “Metabolic reprogramming” in ovarian cancer cells resistant to cisplatin. Curr Cancer Drug Targets 11:226–235. doi:10.2174/156800911794328501
Moreira PI, Custódio J, Moreno A, Oliveira CR, Santos MS (2006) Tamoxifen and estradiol interact with the flavin mononucleotide site of complex I leading to mitochondrial failure. J Biol Chem 281:10143–10152. doi:10.1074/jbc.M510249200
Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, Saavedra E (2007) Energy metabolism in tumor cells. FEBS J 274:1393–1418. doi:10.1111/j.1742-4658.2007.05686.x
Moreno-Sánchez R, Marín-Hernández A, Saavedra E, Pardo JP, Ralph SJ, Rodríguez-Enríquez S (2014) Who controls the ATP supply in cancer cells? Biochemistry lessons to understand cancer energy metabolism. Int J Biochem Cell Biol 2725:42–49. doi:10.1016/j.biocel.2014.01.025
Mrozek E, Kolesar J, Young D, Allen J, Villalona-Calero M, Shapiro CL (2008) Phase II study of sequentially administered low-dose mitomycin-C(MMC) and irinotecan (CPT-11) in women with metastatic breast cancer (MBC). Ann Oncol 19:1417–1422. doi:10.1093/annonc/mdn154
Müller M, Siems W, Buttgereit F, Dumdey R, Rapoport SM (1986) Quantification of ATP-producing and consuming processes of Ehrlich ascites tumour cells. Eur J Biochem 161:701–705. doi:10.1111/j.1432-1033.1986.tb10496.x
Nicolay K, Timmers RJ, Spoelstra E, Van der Neut R, Fok JJ, Huigen YM, Verkleij AJ, De Kruijff B (1984) The interaction of adriamycin with cardiolipin in model and rat liver mitochondrial membranes. Biochim Biophys Acta 778:359–371. doi:10.1016/0005-2736(84)90380-8
Nowis D, Maczewski M, Mackiewicz U, Kujawa M, Ratajska A, Wieckowski MR, Wilczyński GM, Malinowska M, Bil J, Salwa P et al (2010) Cardiotoxicity of the anticancer therapeutic agent bortezomib. Am J Pathol 176:2658–2668. doi:10.2353/ajpath.2010.090690
Ott PA, Adams S (2011) Small-molecule protein kinase inhibitors and their effects on the immune system: implications for cancer treatment. Immunotherapy 3:213–227. doi:10.2217/imt.10.99
Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S (2002) Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci USA 99:1259–1263. doi:10.1073/pnas.241655498
Owen MR, Doran E, Halestrap AP (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348:607–614. doi:10.1042/0264-6021:3480607
Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, Pyles EA, Yancopoulos GD, Stahl N, Wiegand SJ (2012) Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15:171–185. doi:10.1007/s10456-011-9249-6
Papanikolaou V, Iliopoulos D, Dimou I, Dubos S, Kappas C, Kitsiou-Tzeli S, Tsezou A (2011) Survivin regulation by HER2 through NF-κB and c-myc in irradiated breast cancer cells. J Cell Mol Med 15:1542–1550. doi:10.1111/j.1582-4934.2010.01149.x
Park JH, Kim TH (2005) Release of cytochrome c from isolated mitochondria by etoposide. J Biochem Mol Biol 38:619–623. http://europepmc.org/abstract/MED/16202244
Patenaude A, Deschesnes RG, Rousseau JL, Petitclerc E, Lacroix J, Côté MF, C-Gaudreault R (2007) New soft alkylating agents with enhanced cytotoxicity against cancer cells resistant to chemotherapeutics and hypoxia. Cancer Res 67:2306–2316. doi:10.1158/0008-5472.CAN-06-3824
Pathania D, Sechi M, Palomba M, Sanna V, Berretini F, Sias A, Taheri L, Neamati N (2014) Design and discovery of novel quinazolinedione-based redox modulators as therapies for pancreatic cancer. Biochim Biophys Acta 1840:332–343. doi:10.1016/j.bbagen.2013.08.005
Paz MM, Zhang X, Lu J, Holmgren A (2012) A new mechanism of action for the anticancer drug mitomycin C: mechanism-based inhibition of thioredoxin reductase. Chem Res Toxicol 25:1502–1511. doi:10.1021/tx3002065
Piperdi B, Ling YH, Liebes L, Muggia F, Perez-Soler R (2011) Bortezomib: understanding the mechanism of action. Mol Cancer Ther 10:2029–2030. doi:10.1158/1535-7163.MCT-11-0745
Polivka J Jr, Janku F (2014) Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther 142:164–175. doi:10.1016/j.pharmthera.2013.12.004
Pollard PJ, Brière JJ, Alam NA, Barwell J, Barclay E, Wortham NC, Hunt T, Mitchell M, Olpin S, Moat SJ et al (2005) Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet 14:2231–2239. doi:10.1093/hmg/ddi227
Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A (2001) The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer 8:11-31. doi:10.1677/erc.0.0080011
Pritsos CA, Briggs LA, Gustafson DL (1997) A new cellular target for mitomycin C: a case for mitochondrial DNA. Oncol Res 9:333–337. http://www.biomedsearch.com/nih/new-cellular-target-mitomycin-C/9406239.html
Qian W, Nishikawa M, Haque AM, Hirose M, Mashimo M, Sato E, Inoue M (2005) Mitochondrial density determines the cellular sensitivity to cisplatin-induced cell death. Am J Physiol Cell Physiol 289:C1466–C1475. doi:10.1152/ajpcell.00265.2005
Radu M, Semenova G, Kosoff R, Chernoff J (2014) PAK signaling during the development and progression of cancer. Nat Rev Cancer 14:13–25. doi:10.1038/nrc3645
Rix U, Hantschel O, Dürnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J, Köcher T, Superti-Furga G (2007) Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood 110:4055–4063. doi:10.1182/blood-2007-07-102061
Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S (2000) Distinct pathways for stimulation of cytochrome c release by etoposide. J Biol Chem 275:32438–32443. doi:10.1074/jbc.C000518200
Rodríguez-Enríquez S, Torres-Márquez ME, Moreno-Sánchez R (2000) Substrate oxidation and ATP supply in AS-30D hepatoma cells. Arch Biochem Biophys 375:21–30. doi:10.1006/abbi.1999.1582
Rodríguez-Enríquez S, Vital-González PA, Flores-Rodríguez FL, Marín-Hernández A, Ruiz-Azuara L, Moreno-Sánchez R (2006) Control of celullar proliferation by modulation of oxidative phosphorylation in human and rodent fast-growing tumor cells. Toxicol Appl Pharmacol 215:208–217. doi:10.1016/j.taap.2006.02.005
Rodríguez-Enríquez S, Gallardo-Pérez JC, Avilés-Salas A, Marín-Hernández A, Carreño-Fuentes L, Maldonado-Lagunas V, Moreno-Sánchez R (2008) Energy metabolism transition in multi-cellular human tumor spheroids. J Cell Physiol 216:189–197. doi:10.1002/jcp.21392
Rodríguez-Enríquez S, Marín-Hernández A, Gallardo-Pérez JC, Carreño-Fuentes L, Moreno-Sánchez R (2009) Targeting of cancer energy metabolism. Mol Nutr Food Res 53:29–48. doi:10.1002/mnfr.200700470
Rodríguez-Enríquez S, Carreño-Fuentes L, Gallardo-Pérez JC, Saavedra E, Quezada H, Vega A, Marín-Hernández A, Olín-Sandoval V, Torres-Márquez ME, Moreno-Sánchez R (2010) Oxidative phosphorylation is impaired by prolonged hypoxia in breast and possibly in cervix carcinoma. Int J Biochem Cell Biol 42:1744–1751. doi:10.1016/j.biocel.2010.07.010
Rodríguez-Enríquez S, Gallardo-Pérez JC, Marín-Hernández A, Aguilar-Ponce JL, Mandujano-Tinoco EA, Meneses A, Moreno-Sánchez R (2011) Oxidative phosphorylation as a target to arrest malignant neoplasias. Curr Med Chem 18:3156–3167. doi:10.2174/092986711796391561
Rolfe DF, Brown GC (1997) Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77:731–758. http://physrev.physiology.org/content/77/3/731.long
Rumbach L, Warter JM, Rendon A, Marescaux C, Micheletti G, Waksman A (1983) Inhibition of oxidative phosphorylation in hepatic and cerebral mitochondria of sodium valproate-treated rats. J Neurol Sci 61:417–423. doi:10.1016/0022-510X(83)90174-0
Saito T, Kaneko T (1968) Effects of simultaneous application of mitomycin C and prednisolone on respiration and glycolysis of Ehrlich ascites tumor cells in vitro. Tohoku J Exp Med 95:87–106. doi:10.1620/tjem.95.87
Sasaki M, Okamura M, Ideo A, Shimada J, Suzuki F, Ishihara M, Kikuchi H, Kanda Y, Kunii S, Sakagami H (2006) Re-evaluation of tumor-specific cytotoxicity of mitomycin C, bleomycin and peplomycin. Anticancer Res 26:3373–3380. doi:10.1126/scitranslmed.300765
Schmelz K, Wagner M, Dörken B, Tamm I (2005) 5-Aza-2′-deoxycytidine induces p21WAF expression by demethylation of p73 leading to p53-independent apoptosis in myeloid leukemia. Int J Cancer 114:683–695. doi:10.1002/ijc.20797
Schmidt H, Siems W, Müller M, Dumdey R, Rapoport SM (1991) ATP-producing and consuming processes of Ehrlich mouse ascites tumor cells in proliferating and resting phases. Exp Cell Res 94:122–127
Schnekenburger M, Grandjenette C, Ghelfi J, Karius T, Foliguet B, Dicato M, Diederich M (2011) Sustained exposure to the DNA demethylating agent, 2′-deoxy-5-azacytidine, leads to apoptotic cell death in chronic myeloid leukemia by promoting differentiation, senescence, and autophagy. Biochem Pharmacol 81:364–378. doi:10.1016/0014-4827(91)90140-P
Sculier JP, Ghisdal L, Berghmans T, Branle F, Lafitte JJ, Vallot F, Meert AP, Lemaitre F, Steels E, Burniat A et al (2001) The role of mitomycin in the treatment of non-small cell lung cancer: a systematic review with meta-analysis of the literature. Br J Cancer 84:1150–1155. doi:10.1054/bjoc.2001.1742
Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E (2005) Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 7:77–85. doi:10.1016/j.ccr.2004.11.022
Selimovic D, Porzig BB, El-Khattouti A, Badura HE, Ahmad M, Ghanjati F, Santourlidis S, Haikel Y, Hassan M (2013) Bortezomib/proteasome inhibitor triggers both apoptosis and autophagy-dependent pathways in melanoma cells. Cell Signal 25:308–318. doi:10.1016/j.cellsig.2012.10.004
Shah JJ, Kuhn DJ, Orlowski RZ (2009) Bortezomib and EGCG: no green tea for you? Blood 113:5695–5696. doi:10.1182/blood-2009-03-204776
Shin DY, Park YS, Yang K, Kim GY, Kim WJ, Han MH, Kang HS, Choi YH (2012) Decitabine, a DNA methyltransferase inhibitor, induces apoptosis in human leukemia cells through intracellular reactive oxygen species generation. Int J Oncol 41:910–918. doi:10.3892/ijo.2012.1546
Silva MF, Ruiter JP, Illst L, Jakobs C, Duran M, de Almeida IT, Wanders RJ (1997) Valproate inhibits the mitochondrial pyruvate-driven oxidative phosphorylation in vitro. J Inherit Metab Dis 20:397–400. doi:10.1023/A:1005398516208
Singh KK, Russell J, Sigala B, Zhang Y, Williams J, Keshav KF (1999) Mitochondrial DNA determines the cellular response to cancer therapeutic agents. Oncogene 18:6641–6646. doi:10.1038/sj.onc.1203056
Song IS, Kim HK, Lee SR, Jeong SH, Kim N, Ko KS, Rhee BD, Han J (2013) Mitochondrial modulation decreases the bortezomib-resistance in multiple myeloma cells. Int J Cancer 133:1357–1367. doi:10.1002/ijc.28149
Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF et al (2008) Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 118:3930–3942. doi:10.1172/JCI36843
Spanswick VJ, Cummings J, Smyth JF (1996) Enzymology of mitomycin C metabolic activation in tumour tissue. Characterization of a novel mitochondrial reductase. Biochem Pharmacol 51:1623–1630. doi:10.1016/0006-2952(96)00104-9
Surendran S, Krishnamurthy V (2012) Effect of tanmoxifen on mitochondria—an in vitro study. Am J Pharmatech Res 2:471–482. http://www.ajptr.com/archive/volume-2/april-2012-issue-2/article-161.html
Sutherland RM (1988) Cell and environment interactions in tumor microregions: the multicell spheroid model. Science 240:177–184. doi:10.1126/science.2451290
Sviriaeva IV, Ruuge EK, Shumaev KB (2007) Formation of superoxide radicals in isolated cardiac mitochondria: effect of adriamycin. Biofizika 52:1054–1059. doi:10.1134/S0006350910020119
Takai N, Kawamata N, Gui D, Said JW, Miyakawa I, Koeffler HP (2004) Human ovarian carcinoma cells: histone deacetylase inhibitors exhibit antiproliferative activity and potently induce apoptosis. Cancer 101:2760–2770. doi:10.1002/cncr.20709
Tamulevicius P, Streffer C (1995) Metabolic imaging in tumours by means of bioluminescence. Br J Cancer 72:1102–1112. doi:10.1038/bjc.1995.472
Tedder TF, Engel P (1994) CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today 15:450–454. doi:10.1016/0167-5699(94)90276-3
Tello D, Balsa E, Acosta-Iborra B, Fuertes-Yebra E, Elorza A, Ordóñez Á, Corral-Escariz M, Soro I, López-Bernardo E, Perales-Clemente E et al (2011) Induction of the mitochondrial NDUFA4L2 protein by HIF-1α decreases oxygen consumption by inhibiting Complex I activity. Cell Metab 14:768–779. doi:10.1016/j.cmet.2011.10.008
Trojnar MK, Wierzchowska-Cioch E, Krzyzanowski M, Jargiełło M, Czuczwar SJ (2004) New generation of valproic acid. Pol J Pharmacol 56:283–288. http://www.if-pan.krakow.pl/pjp/pdf/2004/3_283.pdf
Tuquet C, Dupont J, Mesneau A, Roussaux J (2000) Effects of tamoxifen on the electron transport chain of isolated rat liver mitochondria. Cell Biol Toxicol 16:207–219. doi:10.1023/A:1007695308257
Vaupel P, Kallinowski F, Okunieff P (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 49:6449–6465. http://cancerres.aacrjournals.org/content/49/23/6449
Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F (2012) Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 122:253–270. doi:10.1042/CS20110386
Von Ahsen O, Waterhouse NJ, Kuwana T, Newmeyer DD, Green DR (2000) The ‘harmless’ release of cytochrome c. Cell Death Differ 7:1192–1199. http://www.nature.com/cdd/journal/v7/n12/pdf/4400782a.pdf
Waller CF (2010) Imatinib mesylate. Recent Results Cancer Res 184:3–20. doi:10.1007/978-3-642-01222-8_1
Wang M, Han XH, Zhang L, Yang J, Qian JF, Shi YK, Kwak LW, Romaguera J, Yi Q (2008) Bortezomib is synergistic with rituximab and cyclophosphamide in inducing apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leukemia 22:179–185. doi:10.1038/sj.leu.2404959
Will Y, Dykens JA, Nadanaciva S, Hirakawa B, Jamieson J, Marroquin LD, Hynes J, Patyna S, Jessen BA (2008) Effect of the multitargeted tyrosine kinase inhibitors imatinib, dasatinib, sunitinib, and sorafenib on mitochondrial function in isolated rat heart mitochondria and H9c2 cells. Toxicol Sci 106:153–161. doi:10.1093/toxsci/kfn157
Williams SA, McConkey DJ (2003) The proteasome inhibitor bortezomib stabilizes a novel active form of p53 in human LNCaP-Pro5 prostate cancer cells. Cancer Res 63:7338–7344. http://cancerres.aacrjournals.org/content/63/21/7338
Xu J, Zhou JY, Tainsky MA, Wu GS (2007) Evidence that tumor necrosis factor-related apoptosis-inducing ligand induction by 5-Aza-2′-deoxycytidine sensitizes human breast cancer cells to adriamycin. Cancer Res 67:1203–1211. doi:10.1158/0008-5472.CAN-06-2310
Xu J, Wang J, Xu B, Ge H, Zhou X, Fang JY (2013) Colorectal cancer cells refractory to anti-VEGF treatment are vulnerable to glycolytic blockade due to persistent impairment of mitochondria. Mol Cancer Ther 12:717–724. doi:10.1158/1535-7163.MCT-12-1016-T
Yang Z, Schumaker LM, Egorin MJ, Zuhowski EG, Guo Z, Cullen KJ (2006) Cisplatin preferentially binds mitochondrial DNA and voltage-dependent anion channel protein in the mitochondrial membrane of head and neck squamous cell carcinoma: possible role in apoptosis. Clin Cancer Res 12:5817–5825. doi:10.1158/1078-0432.CCR-06-1037
Yaromina A, Meyer S, Fabian C, Zaleska K, Sattler UG, Kunz-Schughart LA, Mueller-Klieser W, Zips D, Baumann M (2012) Effects of three modifiers of glycolysis on ATP, lactate, hypoxia, and growth in human tumor cell lines in vivo. Strahlenther Onkol 188:431–437. doi:10.1007/s00066-011-0054-3
Yin D, Zhou H, Kumagai T, Liu G, Ong JM, Black KL, Koeffler HP (2005) Proteasome inhibitor PS-341 causes cell growth arrest and apoptosis in human glioblastoma multiforme (GBM). Oncogene 24:344–354. doi:10.1038/sj.onc.1208225
Yu D, Hung MC (2000) Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene 19:6115–6121. doi:10.1038/sj.onc.1203972
Zgouras D, Becker U, Loitsch S, Stein J (2004) Modulation of angiogenesis-related protein synthesis by valproic acid. Biochem Biophys Res Commun 316:693–697. doi:10.1016/j.bbrc.2004.02.105
Zhang D, Tai LK, Wong LL, Chiu LL, Sethi SK, Koay ES (2005) Proteomic study reveals that proteins involved in metabolic and detoxification pathways are highly expressed in HER-2/neu-positive breast cancer. Mol Cell Proteomics 4:1686–1696. doi:10.1074/mcp.M400221-MCP200
Zhao F, Mancuso A, Bui TV, Tong X, Gruber JJ, Swider CR, Sanchez PV, Lum JJ, Sayed N, Melo JV et al (2010) Imatinib resistance associated with BCR-ABL upregulation is dependent on HIF-1alpha-induced metabolic reprograming. Oncogene 29:2962–2972. doi:10.1038/onc.2010.67
Zhao Y, Liu H, Liu Z, Ding Y, Ledoux SP, Wilson GL, Voellmy R, Lin Y, Lin W, Nahta R, Liu B, Fodstad O, Chen J, Wu Y, Price JE, Tan M (2011) Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res 71:4585–4597. doi:10.1158/0008-5472.CAN-11-0127
Zhao L, Ashek A, Wang L, Fang W, Dabral S, Dubois O, Cupitt J, Pullamsetti SS, Cotroneo E, Jones H et al (2013) Heterogeneity in lung (18)FDG uptake in pulmonary arterial hypertension: potential of dynamic (18)FDG positron emission tomography with kinetic analysis as a bridging biomarker for pulmonary vascular remodeling targeted treatments. Circulation 128:1214–1224. doi:10.1161/CIRCULATIONAHA.113.004136
Zsengellér ZK, Ellezian L, Brown D, Horváth B, Mukhopadhyay P, Kalyanaraman B, Parikh SM, Karumanchi SA, Stillman IE, Pacher P (2012) Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J Histochem Cytochem 60:521–529. doi:10.1369/0022155412446227
Zu XL, Guppy M (2004) Cancer metabolism: facts, fantasy, and fiction. Biochem Biophys Res Commun 313:459–465. doi:10.1016/j.bbrc.2003.11.136
Acknowledgments
The present work was partially supported by CONACyT-México Grant Nos. 107183 to SRE, 180322 to AMH and 80534 and 123636 to RMS; and Instituto de Ciencia y Tecnología del Distrito Federal Grant No. PICS08.
Conflict of interest
The authors declare there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rodríguez-Enríquez, S., Gallardo-Pérez, J.C., Hernández-Reséndiz, I. et al. Canonical and new generation anticancer drugs also target energy metabolism. Arch Toxicol 88, 1327–1350 (2014). https://doi.org/10.1007/s00204-014-1246-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-014-1246-2