Abstract
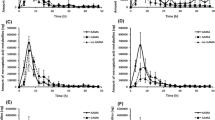
Acrylamide (AA), classified as class 2A carcinogen (probably carcinogenic to humans) by the International Agency for Research on Cancer (IARC), is formed during heating of food from reducing carbohydrates and asparagine by Maillard reaction chemistry. After dietary uptake, AA is in part metabolically converted into the proximate genotoxic phase I metabolite glycidamide (GA). GA reacts with nucleophilic base positions in DNA, primarily forming N7-(2-carbamoyl-2-hydroxyethyl)guanine (N7-GA-Gua) adducts. In a competing phase II biotransformation pathway AA, as well as its phase I metabolite GA, is coupled to glutathione (GSH). The GSH coupling products are further biotransformed and excreted via urine as mercapturic acids (MA), N-acetyl-S-(2-carbamoylethyl)cysteine (AAMA), and N-acetyl-S-(2-hydroxy-2-carbamoylethyl)cysteine (GAMA). In the present study, hepatic biotransformation pathways and DNA adduct formation were studied in primary rat hepatocytes, incubated with AA (0.2–2,000 μM) for up to 24 h. Contents of AA-GSH, GA, AAMA, and GAMA were measured in the cell culture medium after solid phase extraction (SPE). N7-GA-Gua adducts in DNA of hepatocytes were determined by HPLC–ESI–MS/MS after lysis of the cells and neutral thermal hydrolysis. Formation of AA-GSH was linear with AA concentration and incubation time and became detectable already at 0.2 μM (4 h). In contrast to AA, GA was not detected before 16 h incubation at 10-fold higher AA concentration (2 μM). In summary, the rate of AA-GSH formation was found to be about 1.5–3 times higher than that of GA formation. N7-GA-Gua adducts were found only at the highest AA concentration tested (2,000 μM).






Similar content being viewed by others
References
Barlow S, Renwick AG, Kleiner J et al (2006) Risk assessment of substances that are both genotoxic and carcinogenic report of an International Conference organized by EFSA and WHO with support of ILSI Europe. Food Chem Toxicol 44(10):1636–1650. doi:10.1016/j.fct.2006.06.020
Baum M, Fauth E, Fritzen S et al (2005) Acrylamide and glycidamide: approach towards risk assessment based on biomarker guided dosimetry of genotoxic/mutagenic effects in human blood. Adv Exp Med Biol 561:77–88. doi:10.1007/0-387-24980-X_6
Berger FI, Feld J, Bertow D et al (2011) Biological effects of acrylamide after daily ingestion of various foods in comparison to water: a study in rats. Mol Nutr Food Res 55(3):387–399. doi:10.1002/mnfr.201000234
Boettcher MI, Angerer J (2005) Determination of the major mercapturic acids of acrylamide and glycidamide in human urine by LC-ESI-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 824(1–2):283–294. doi:10.1016/j.jchromb.2005.07.042
Boettcher MI, Bolt HM, Drexler H, Angerer J (2006) Excretion of mercapturic acids of acrylamide and glycidamide in human urine after single oral administration of deuterium-labelled acrylamide. Arch Toxicol 80(2):55–61. doi:10.1007/s00204-005-0011-y
Boon PE, de Mul A, van der Voet H, van Donkersgoed G, Brette M, van Klaveren JD (2005) Calculations of dietary exposure to acrylamide. Mutat Res 580(1–2):143–155. doi:10.1016/j.mrgentox.2004.10.014
Citti L, Gervasi PG, Turchi G, Bellucci G, Bianchini R (1984) The reaction of 3,4-epoxy-1-butene with deoxyguanosine and DNA in vitro: synthesis and characterization of the main adducts. Carcinogenesis 5(1):47–52
Doerge DR, Young JF, McDaniel LP, Twaddle NC, Churchwell MI (2005) Toxicokinetics of acrylamide and glycidamide in Fischer 344 rats. Toxicol Appl Pharmacol 208(3):199–209. doi:10.1016/j.taap.2005.03.003
EFSA (2011) Results on acrylamide levels in food from monitoring years 2007–2009 and exposure assessment. EFSA J 9(4):2133–48
Fuhr U, Boettcher MI, Kinzig-Schippers M et al (2006) Toxicokinetics of acrylamide in humans after ingestion of a defined dose in a test meal to improve risk assessment for acrylamide carcinogenicity. Cancer Epidemiol Biomarkers Prev 15(2):266–271. doi:10.1158/1055-9965.EPI-05-0647
Gamboa da Costa G, Churchwell MI, Hamilton LP et al (2003) DNA adduct formation from acrylamide via conversion to glycidamide in adult and neonatal mice. Chem Res Toxicol 16(10):1328–1337. doi:10.1021/tx034108e
Giannerini F, Giustarini D, Lusini L, Rossi R, Di Simplicio P (2001) Responses of thiols to an oxidant challenge: differences between blood and tissues in the rat. Chem Biol Interact 134(1):73–85
Hinchman CA, Ballatori N (1990) Glutathione-degrading capacities of liver and kidney in different species. Biochem Pharmacol 40(5):1131–1135
Hinchman CA, Ballatori N (1994) Glutathione conjugation and conversion to mercapturic acids can occur as an intrahepatic process. J Toxicol Environ Health 41(4):387–409. doi:10.1080/15287399409531852
Hinchman CA, Matsumoto H, Simmons TW, Ballatori N (1991) Intrahepatic conversion of a glutathione conjugate to its mercapturic acid. Metabolism of 1-chloro-2,4-dinitrobenzene in isolated perfused rat and guinea pig livers. J Biol Chem 266(33):22179–22185
JECFA Summary report of the seventy-second meeting of JECFA. In: 72 meeting, Rome, 2010. JOINT FAO/WHO EXPERT COMMITTEE ON FOOD ADDITIVES
Kedderis GL (1996) Biochemical basis of hepatocellular injury. Toxicol Pathol 24(1):77–83
Kurebayashi H, Ohno Y (2006) Metabolism of acrylamide to glycidamide and their cytotoxicity in isolated rat hepatocytes: protective effects of GSH precursors. Arch Toxicol 80(12):820–828. doi:10.1007/s00204-006-0109-x
Langley-Evans SC, Phillips GJ, Jackson AA (1996) Sulphur dioxide: a potent glutathione depleting agent. Comp Biochem Physiol C: Pharmacol Toxicol Endocrinol 114(2):89–98
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760. doi:10.1146/annurev.bi.52.070183.003431
O’Brien J, Wilson I, Orton T, Pognan F (2000) Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 267(17):5421–5426
O’Brien J, Renwick AG, Constable A et al (2006) Approaches to the risk assessment of genotoxic carcinogens in food: a critical appraisal. Food Chem Toxicol 44(10):1613–1635. doi:10.1016/j.fct.2006.07.004
Pernice R, Hauder J, Koehler P, Vitaglione P, Fogliano V, Somoza V (2009) Effect of sulforaphane on glutathione-adduct formation and on glutathione_S_transferase-dependent detoxification of acrylamide in Caco-2 cells. Mol Nutr Food Res 53(12):1540–1550. doi:10.1002/mnfr.200900447
Puppel N, Tjaden Z, Fueller F, Marko D (2005) DNA strand breaking capacity of acrylamide and glycidamide in mammalian cells. Mutat Res 580(1–2):71–80. doi:10.1016/j.mrgentox.2004.11.009
Rebbeor JF, Wang W, Clifton D, Ballatori N (1998) Glutathione S-conjugate formation and metabolism in HepG2 cells: a cell model of mercapturic acid biosynthesis. J Toxicol Environ Health A 53(8):651–663
Roe R, Paul JS, Montgomery POB (1973) Synthesis and PMR spectra of 7-hydroxyalkylguanosinium acetates. J Heterocycl Chem 10(5):849–857
Schug M, Heise T, Bauer A et al (2008) Primary rat hepatocytes as in vitro system for gene expression studies: comparison of sandwich, Matrigel and 2D cultures. Arch Toxicol 82(12):923–931. doi:10.1007/s00204-008-0375-x
Segerback D, Calleman CJ, Schroeder JL, Costa LG, Faustman EM (1995) Formation of N-7-(2-carbamoyl-2-hydroxyethyl)guanine in DNA of the mouse and the rat following intraperitoneal administration of [14C]acrylamide. Carcinogenesis 16(5):1161–1165
Shaw LM, London JW, Petersen LE (1978) Isolation of gamma-glutamyltransferase from human liver, and comparison with the enzyme from human kidney. Clin Chem 24(6):905–915
Smith CJ, Perfetti TA, Mullens MA, Rodgman A, Doolittle DJ (2000) “IARC group 2A Carcinogens” reported in cigarette mainstream smoke. Food Chem Toxicol 38(9):825–848
Sumner SC, Fennell TR, Moore TA, Chanas B, Gonzalez F, Ghanayem BI (1999) Role of cytochrome P450 2E1 in the metabolism of acrylamide and acrylonitrile in mice. Chem Res Toxicol 12(11):1110–1116
Tarun M, Rusling JF (2005) Quantitative measurement of DNA adducts using neutral hydrolysis and LC-MS. Validation of genotoxicity sensors. Anal Chem 77(7):2056–2062. doi:10.1021/ac048283r
Thielen S, Baum M, Hoffmann M, Loeppky RN, Eisenbrand G (2006) Genotoxicity of glycidamide in comparison to (±)-anti-benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide and alpha-acetoxy-N-nitroso-diethanolamine in human blood and in mammalian V79-cells. Mol Nutr Food Res 50(4–5):430–436. doi:10.1002/mnfr.200500227
Watzek N, Bohm N, Feld J et al (2012a) N7-glycidamide-guanine DNA adduct formation by orally ingested acrylamide in rats: a dose-response study encompassing human diet-related exposure levels. Chem Res Toxicol 25(2):381–390. doi:10.1021/tx200446z
Watzek N, Scherbl D, Feld J et al (2012b) Profiling of mercapturic acids of acrolein and acrylamide in human urine after consumption of potato crisps. Mol Nutr Food Res 56(12):1825–1837. doi:10.1002/mnfr.201200323
Zerilli A, Lucas D, Amet Y et al (1995) Cytochrome P-450 2E1 in rat liver, kidney and lung microsomes after chronic administration of ethanol either orally or by inhalation. Alcohol Alcohol 30(3):357–365
Zyzak DV, Sanders RA, Stojanovic M et al (2003) Acrylamide formation mechanism in heated foods. J Agric Food Chem 51(16):4782–4787. doi:10.1021/jf034180i
Acknowledgments
This study was supported by ISIC (Institute for Scientific Information on Coffee, La Tour de Peilz, Switzerland). The authors thank Julia Feld and Franz Berger for generously sharing their experience with us and Karl-Heinz Merz for his help with the synthesis of reference compounds. The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watzek, N., Scherbl, D., Schug, M. et al. Toxicokinetics of acrylamide in primary rat hepatocytes: coupling to glutathione is faster than conversion to glycidamide. Arch Toxicol 87, 1545–1556 (2013). https://doi.org/10.1007/s00204-013-1054-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-013-1054-0