Abstract
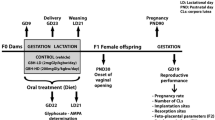
Sexual differentiation in the brain takes place from late gestation to the early postnatal days. This is dependent on the conversion of circulating testosterone into estradiol by the enzyme aromatase. The glyphosate was shown to alter aromatase activity and decrease serum testosterone concentrations. Thus, the aim of this study was to investigate the effect of gestational maternal glyphosate exposure (50 mg/kg, NOAEL for reproductive toxicity) on the reproductive development of male offspring. Sixty-day-old male rat offspring were evaluated for sexual behavior and partner preference; serum testosterone concentrations, estradiol, FSH and LH; the mRNA and protein content of LH and FSH; sperm production and the morphology of the seminiferous epithelium; and the weight of the testes, epididymis and seminal vesicles. The growth, the weight and age at puberty of the animals were also recorded to evaluate the effect of the treatment. The most important findings were increases in sexual partner preference scores and the latency time to the first mount; testosterone and estradiol serum concentrations; the mRNA expression and protein content in the pituitary gland and the serum concentration of LH; sperm production and reserves; and the height of the germinal epithelium of seminiferous tubules. We also observed an early onset of puberty but no effect on the body growth in these animals. These results suggest that maternal exposure to glyphosate disturbed the masculinization process and promoted behavioral changes and histological and endocrine problems in reproductive parameters. These changes associated with the hypersecretion of androgens increased gonadal activity and sperm production.





Similar content being viewed by others
References
Almiron I, Chemes H (1988) Spermatogenic onset. II. FSH modulates mitotic activity of germ and sertoli cells in immature rats. Int J Androl 11:235–246
Bakker J, Honda SI, Harada N, Balthazart J (2002) The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci 22:9104–9112
Bancoft J (2005) The endocrinology of sexual arousal. J Endocrinol 186:411–427
Bardin CW, Catterall JF (1981) Testosterone: a major determinant of extragenital sexual dimorphism. Science 211:1285–1294
Benachour N, Seralini GE (2009) Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem Res Toxicol 22:97–105
Benachour N, Sipahutar H, Moslemi S, Gasnier C, Travert C, Seralini GE (2007) Time and dose-dependent effects of roundup on human embryonic and placental cells. Arch Environ Contam Toxicol 53:126–133
Bradford MM (1976) Bradford a rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cerdeira AL, Gazziero DLP, Duke SO, Matallo MB, Spadotto CA (2007) Review of potential environmental impacts of transgenic glyphosato-resistent soybean in Brazil. J Environ Sci Health B 42:539–549
Chiavegatto S, Bernardi MM, De-Souza-Spinosa H (1989) Effects of prenatal diphenhydramine administration on sexual behavior of rats. Braz J Med Biol Res 22:729–732
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Dahlgren IL, Matuszczyk JV, Hård E (1991) Sexual orientation in male rats prenatally exposed to ethanol. Neurotoxicol Teratol 3:267–269
Dallegrave E, Mantese FD, Oliveira RT, Andrade AJM, Dalsenter PR, Langeloh A (2007) Pre- and postnatal toxicity of the commercial glyphosate formulation in Wistar rats. Arch Toxicol 81:665–673
Felicio LF, Palermo-Neto J, Nasello AG (1989) Perinatal bromopride treatment: effects on sexual behavior of male and female rats. Behav Neural Biol 52:145–151
França RL, Silva VA Jr, Chiarini-Garcia H, Garcia SK, Debeljuk L (2000) Cell proliferation and hormonal changes during postnatal development of the testis in the pig. Biol Reprod 63:1629–1636
Gasnier C, Dumont C, Nora Benachour N, Clair E, Chagnon MC, Seralini GE (2009) Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 262:184–191
Gerardin DC, Pereira OC, Kempinas WG, Florio JC, Moreira EG, Bernardi MM (2005) Sexual behavior, neuroendocrine, and neurochemical aspects in male rats exposed prenatally to stress. Physiol Behav 84:97–104
Goulart-Silva F, de Souza PB, Nunes MT (2011) T3 rapidly modulates TSHβ mRNA stability and translational rate in the pituitary of hypothyroid rats. Mol Cell Endocrinol 332:277–282
Harding SM, McGinnis MY (2003) Effects of testosterone in the VMN on copulation, partner preference, and vocalizations in male rats. Horm Behav 43:327–335
Harding SM, McGinnis MY (2004) Androgen receptor blockade in the MPOA or VMN: effects on male sociosexual behaviors. Physiol Behav 81:671–680
Harding SM, McGinnis MY (2005) Microlesions of the ventromedial nucleus of the hypothalamus: effects on sociosexual behaviors in male rats. Behav Neurosci 119:1227–1234
Harding SM, Velotta JP (2011) Comparing the relative amount of testosterone required to restore sexual arousal, motivation, and performance in male rats. Horm Behav 59:666–673
Hayes WJ, Laws ER (1991) Handbook of pesticide toxicology. Academic Press, San Diego
Heindel JJ, Kimberley AT (1989) Physiology of the male reproductive system: paracrine and autocrine regulation. Toxicol Pathol 17:411–445
Hoepfner BA, Ward IL (1988) Prenatal and neonatal androgen exposure interact to affect sexual differentiation in female rats. Behav Neurosci 102:61–65
Inoue MH, Oliveira-Jr RS, Regitano JB, Tormena CA, Tornisielo VL, Constantin J (2003) Critérios para avaliação do potencial de lixiviação dos herbicidas comercializados no Estado do Paraná. Planta Daninha 21:313–323
Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA (1996) Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect 104:715–740
Knudsen JF, Max SR (1980) Aromatization of androgens to estrogens mediates increased activity of glucose 6-phosphate dehydrogenase in rat levator ani muscle. Endocrinology 106:440–443
Korenbrot CC, Huhtaniemi IT, Weiner RI (1977) Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod 17:298–303
Lenz KM, McCarthy MM (2010) Organized for sex–steroid hormones and the developing hypothalamus. Eur J Neurosci 32:2096–2104
Lu FC (1995) A review of the acceptable daily intakes of pesticides assessed by WHO. Regul Toxicol Pharmacol 21:352–364
MacLusky NJ, Naftolin F (1981) Sexual differentiation of the central nervous system. Science 211:1294–1302
Max SR, Knudsen JF (1980) Neural and endocrine interaction in skeletal muscle. Brain Res 26:49–553
Meisel RL, Sachs BD (1994) The physiology of male sexual behavior. In: Knobil E, Neill D (eds) The physiology of reproduction, 2nd edn. Raven Press, New York, pp 3–105
Mendelson SD, Pfaus JG (1989) Level searching: a new assay of sexual motivation in the male rat. Physiol Behav 45:337–341
Mruk DD, Yan Cheng C (2004) Sertoli-sertoli and sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 25:747–806
Nolan T, Hands RE, Bustin SA (2006) Quantification of mRNA using real-time RT-PCR. Nat Protoc 1:1559–1582
Orth JM, Gunsalus GL, Lamperti AA (1988) Evidence from sertoli-cell depleted rats indicates that spermatid number in adults depends on numbers of sertoli cells produced during perinatal development. Endocrinology 122:787–794
Pereira OC, Bernardi MM, Gerardin DC (2006) Could neonatal testosterone replacement prevent alterations induced by prenatal stress in male rats? Life Sci 78:2767–2771
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Pfaus JG, Kippin TE, Centeno S (2001) Conditioning and sexual behavior: a review. Horm Behav 40:291–321
Phoenix HC, Goy RW, Gerall AA, Young W (1959) Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369–382
Piffer RC, Garcia PC, Gerardin DC, Kempinas WG, Pereira OC (2009) Semen parameters, fertility and testosterone levels in male rats exposed prenatally to betamethasone. Reprod Fertil Dev 21:634–639
Reznikov AG, Tarasenko LV (2007) Hormonal protection of gender-related peculiarities of testosterone metabolism in the brain of prenatally stressed rats. Neuro Endocrinol Lett 28:671–674
Richard S, Moslemi S, Sipahutar H, Benachour N, Seralini G (2005) Differential effects of glyphosate and roundup on human placental cells. Environ Health Perspect 113:716–720
Robb GW, Amann RP, Killian GJ (1978) Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J Reprod Fert 54:103–107
Romano RM, Romano MA, Bernardi MM, Furtado PV, Oliveira CA (2010) Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology. Arch Toxicol 84:309–317
Sachs BD (2007) A contextual definition of male sexual arousal. Horm Behav 51:569–578
Saito TR, Moltz H (1986) Copulatory behavior of sexually naive and experienced male rats following removal of the vomeronasal organ. Physiol Behav 37:507–510
Sakuma Y (2009) Gonadal steroid action and brain sex differentiation in the rat. J Neuroendocrinol 21:410–414
Sharpe RM (2001) Hormones and testis development and the possible adverse effects of environmental chemicals. Toxicol Lett 120:221–232
Silva VA Jr, Vieira ACS, Pinto CF, Paula TAR, Palma MB, Amorim MJAAL, Amorim AA Jr, Castro RM (2006) Neonatal treatment with naloxone increases the population of sertoli cells and sperm production in adult rats. Reprod Nutr Dev 46:157–166
Stoker TE, Parks LG, Gray LE, Cooper RL (2000) Endocrine-disrupting chemicals: pubertal exposures and effects on sexual maturation and thyroid function in the male rat. A focus on the EDSTAC recommendations. Endocrine disrupting screening and testing advisory committee. Crit Rev Toxicol 30:197–252
Walsh LP, McCormick C, Martin C, Stocco DM (2000) Roundup inhibits steroidogenesis by disrupting steroidogenic acute regulatory (StAR) protein expression. Environ Health Perspect 108:769–776
Ward IL, Weisz J (1980) Maternal stress alters plasma testosterone in fetal males. Science 207:328–329
Weisz J, Ward IL (1980) Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology 106:306–316
Wilson CA, Davies DC (2007) The control of sexual differentiation of the reproductive system and brain. Reproduction 133:331–359
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (151485/2010-0 to M.A.R.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romano, M.A., Romano, R.M., Santos, L.D. et al. Glyphosate impairs male offspring reproductive development by disrupting gonadotropin expression. Arch Toxicol 86, 663–673 (2012). https://doi.org/10.1007/s00204-011-0788-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-011-0788-9