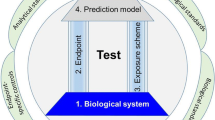
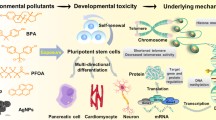
Abstract
Modern society faces an inherent dilemma. In our globalized society, we are spoilt for choice by an ever-increasing number of products, many of which are made of new materials and compound mixtures. At the same time, as consumers we got accustomed to the idea of a life minimized for risk, including our own exposure to chemicals from the environment or to compounds present in and released from everyday products. Chemical safety testing bridges these obviously diverging interests, and the corresponding legislation has hence been tremendously extended (e.g., introduction of the European legislation REACH in 2007). However, the underlying regulatory toxicology still relies mainly on animal testing, which is relatively slow, expensive, and ethically arguable. Meanwhile, recent years have seen a surge in efforts to develop alternative testing systems and strategies. Expectations are particularly high for the applicability of stem cells as test systems especially for developmental toxicity testing in vitro. For the first time in history, test systems can be based on differentiating cells and tissue progenitors in culture, thus bringing the ‘vision of toxicity testing in the 21st century’ a step closer.





Similar content being viewed by others
References
Adler S, Lindqvist J, Uddenberg K, Hyllner J, Strehl R (2008) Testing potential developmental toxicants with a cytotoxicity assay based on human embryonic stem cells. Altern Lab Anim 36:129–140
Andersen ME, Krewski D (2009) Toxicity testing in the 21st century: bringing the vision to life. Toxicol Sci 107:324–330
Andersen ME, Krewski D (2010) The vision of toxicity testing in the 21st century: moving from discussion to action. Toxicol Sci 117:17–24
Baharvand H, Fathi A, van Hoof D, Salekdeh GH (2007) Concise review: trends in stem cell proteomics. Stem Cells 25:1888–1903
Bal-Price AK, Hogberg HT, Buzanska L, Coecke S (2010) Relevance of in vitro neurotoxicity testing for regulatory requirements: challenges to be considered. Neurotoxicol Teratol 32:36–41
Banach K, Halbach MD, Hu P, Hescheler J, Egert U (2003) Development of electrical activity in cardiac myocyte aggregates derived from mouse embryonic stem cells. Am J Physiol Heart Circ Physiol 284:H2114–H2123
Bantle JA, Finch RA, Fort DJ, Stover EL, Hull M, Kumsher-King M, Gaudet-Hull AM (1999) Phase III interlaboratory study of FETAX. Part 3. FETAX validation using 12 compounds with and without an exogenous metabolic activation system. J Appl Toxicol 19:447–472
Bao S, Tang F, Li X, Hayashi K, Gillich A, Lao K, Surani MA (2009) Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature 461:1292–1295
Breier JM, Gassmann K, Kayser R, Stegeman H, de Groot D, Fritsche E, Shafer TJ (2010) Neural progenitor cells as models for high-throughput screens of developmental neurotoxicity: state of the science. Neurotoxicol Teratol 32:4–15
Buesen R, Genschow E, Slawik B, Visan A, Spielmann H, Luch A, Seiler A (2009) Embryonic stem cell test remastered: comparison between the validated EST and the new molecular FACS-EST for assessing developmental toxicity in vitro. Toxicol Sci 108:389–400
Castoldi AF, Johansson C, Onishchenko N, Coccini T, Roda E, Vahter M, Ceccatelli S, Manzo L (2008) Human developmental neurotoxicity of methylmercury: impact of variables and risk modifiers. Regul Toxicol Pharmacol 51:201–214
Cezar GG, Quam JA, Smith AM, Rosa GJ, Piekarczyk MS, Brown JF, Gage FH, Muotri AR (2007) Identification of small molecules from human embryonic stem cells using metabolomics. Stem Cells Dev 16:869–882
Chapin RE, Stedman DB (2009) Endless possibilities: stem cells and the vision for toxicology testing in the 21st century. Toxicol Sci 112:17–22
Chen L, Deng CX (2005) Roles of FGF signaling in skeletal development and human genetic diseases. Front Biosci 10:1961–1976
Coecke S, Balls M, Bowe G, Davis J, Gstraunthaler G, Hartung T, Hay R, Merten OW, Price A, Schechtman L, Stacey G, Stokes W (2005) Guidance on good cell culture practice. A report of the second ECVAM task force on good cell culture practice. Altern Lab Anim 33:261–287
Coecke S, Ahr H, Blaauboer BJ, Bremer S, Casati S, Castell J, Combes R, Corvi R, Crespi CL, Cunningham ML, Elaut G, Eletti B, Freidig A, Gennari A, Ghersi-Egea JF, Guillouzo A, Hartung T, Hoet P, Ingelman-Sundberg M, Munn S, Janssens W, Ladstetter B, Leahy D, Long A, Meneguz A, Monshouwer M, Morath S, Nagelkerke F, Pelkonen O, Ponti J, Prieto P, Richert L, Sabbioni E, Schaack B, Steiling W, Testai E, Vericat JA, Worth A (2006) Metabolism: a bottleneck in in vitro toxicological test development. The report and recommendations of ECVAM workshop 54. Altern Lab Anim 34:49–84
Coecke S, Goldberg AM, Allen S, Buzanska L, Calamandrei G, Crofton K, Hareng L, Hartung T, Knaut H, Honegger P, Jacobs M, Lein P, Li A, Mundy W, Owen D, Schneider S, Silbergeld E, Reum T, Trnovec T, Monnet-Tschudi F, Bal-Price A (2007) Workgroup report: incorporating in vitro alternative methods for developmental neurotoxicity into international hazard and risk assessment strategies. Environ Health Perspect 115:924–931
Collins FS, Gray GM, Bucher JR (2008) Toxicology. Transforming environmental health protection. Science 319:906–907
Combes R, Barratt M, Balls M (2006) An overall strategy for the testing of chemicals for human hazard and risk assessment under the EU REACH system. Altern Lab Anim 34:15–27
Daston GP, Naciff JM (2010) Predicting developmental toxicity through toxicogenomics. Birth Defects Res C Embryo Today 90:110–117
Davis LA, Zur Nieden NI (2008) Mesodermal fate decisions of a stem cell: the Wnt switch. Cell Mol Life Sci 65:2658–2674
de Robertis EM (2008) Evo-devo: variations on ancestral themes. Cell 132:185–195
Desbordes SC, Placantonakis DG, Ciro A, Socci ND, Lee G, Djaballah H, Studer L (2008) High-throughput screening assay for the identification of compounds regulating self-renewal and differentiation in human embryonic stem cells. Cell Stem Cell 2:602–612
Dix DJ, Houck KA, Martin MT, Richard AM, Setzer RW, Kavlock RJ (2007) The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol Sci 95:5–12
Doehmer J, Buters JT, Luch A, Soballa V, Baird WM, Morisson H, Stegeman JJ, Townsend AJ, Greenlee WF, Glatt HR, Seidel A, Jacob J, Greim H (1999) Molecular studies on the toxifying effects by genetically engineered cytochromes P450. Drug Metab Rev 31:423–435
Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R (1985) The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol 87:27–45
European Commission (2003) European parliament and council directive 2003/15/EC. Off J Eur Union L 66:26–35
Erwin DH, Davidson EH (2009) The evolution of hierarchical gene regulatory networks. Nat Rev Genet 10:141–148
European Commission (2007) Fifth report on the statistics on the number of animals used for experimental and other scientific purposes in the member states of the European Union. European Commission, Brussels
European Commission (2010) Sixth report on the statistics on the number of animals used for experimental and other scientific purposes in the member states of the European Union. European Commission, Brussels
Fernandes TG, Diogo MM, Clark DS, Dordick JS, Cabral JM (2009) High-throughput cellular microarray platforms: applications in drug discovery, toxicology and stem cell research. Trends Biotechnol 27:342–349
Festag M, Viertel B, Steinberg P, Sehner C (2007) An in vitro embryotoxicity assay based on the disturbance of the differentiation of murine embryonic stem cells into endothelial cells. II. Testing of compounds. Toxicol In Vitro 21:1631–1640
Genschow E, Spielmann H, Scholz G, Seiler A, Brown N, Piersma A, Brady M, Clemann N, Huuskonen H, Paillard F, Bremer S, Becker K (2002) The ECVAM international validation study on in vitro embryotoxicity tests: results of the definitive phase and evaluation of prediction models. European centre for the validation of alternative methods. Altern Lab Anim 30:151–176
Genschow E, Spielmann H, Scholz G, Pohl I, Seiler A, Clemann N, Bremer S, Becker K (2004) Validation of the embryonic stem cell test in the international ECVAM validation study on three in vitro embryotoxicity tests. Altern Lab Anim 32:209–244
Gilbert SF (2003) Developmental biology. Sinauer Associated, Sunderland
Harrill AH, Rusyn I (2008) Systems biology and functional genomics approaches for the identification of cellular responses to drug toxicity. Expert Opin Drug Metab Toxicol 4:1379–1389
Hartung T (2009) Toxicology for the twenty-first century. Nature 460:208–212
Hartung T (2010) Lessons learned from alternative methods and their validation for a new toxicology in the 21st century. J Toxicol Environ Health B Crit Rev 13:277–290
Hay DC, Zhao D, Fletcher J, Hewitt ZA, McLean D, Urruticoechea-Uriguen A, Black JR, Elcombe C, Ross JA, Wolf R, Cui W (2008) Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells 26:894–902
Hayes B, Fagerlie SR, Ramakrishnan A, Baran S, Harkey M, Graf L, Bar M, Bendoraite A, Tewari M, Torok-Storb B (2008) Derivation, characterization, and in vitro differentiation of canine embryonic stem cells. Stem Cells 26:465–473
Höfer T, Gerner I, Gundert-Remy U, Liebsch M, Schulte A, Spielmann H, Vogel R, Wettig K (2004) Animal testing and alternative approaches for the human health risk assessment under the proposed new European chemicals regulation. Arch Toxicol 78:549–564
Horn PA, Tani K, Martin U, Niemann H (2006) Nonhuman primates: embryonic stem cells and transgenesis. Clon Stem Cells 8:124–129
Huang R, Southall N, Cho MH, Xia M, Inglese J, Austin CP (2008) Characterization of diversity in toxicity mechanism using in vitro cytotoxicity assays in quantitative high throughput screening. Chem Res Toxicol 21:659–667
Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE (2010) Reconstituting organ-level lung functions on a chip. Science 328:1662–1668
Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP (2006) Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci USA 103:11473–11478
Jelinek R, Marhan O (1994) Validation of the chick embryotoxicity screening test (CHEST). A comparative study. Funct Dev Morphol 4:317–323
Joutel A, Tournier-Lasserve E (1998) Notch signalling pathway and human diseases. Semin Cell Dev Biol 9:619–625
Judson RS, Kavlock RJ, Setzer RW, Cohen Hubal EA, Martin MT, Knudsen TB, Houck KA, Thomas RS, Wetmore BA, Dix DJ (2011) Estimating toxicity-related biological pathway altering doses for high-throughput chemical risk assessment. Chem Res Toxicol 24:451–462
Kirschstein R, Skirboll L (2001) Stem cells: scientific progress and future research directions. National Institutes of Health, Bethesda
Knight A (2007) Systematic reviews of animal experiments demonstrate poor human clinical and toxicological utility. Altern Lab Anim 35:641–659
Krewski D, Westphal M, Al-Zoughool M, Croteau MC, Andersen ME (2011) New directions in toxicity testing. Annu Rev Public Health 32:161–178
Landsiedel R, Fabian E, Tralau T, Luch A (2011) Chemical toxicity testing in vitro using P450 expressing cell lines such as human CYP1B1. Nat Protoc 6:677–688
Larsen JW Jr, Greendale K (1985) ACOG technical bulletin number 84-February 1985: teratology. Teratology 32:493–496
Lee MY, Kumar RA, Sukumaran SM, Hogg MG, Clark DS, Dordick JS (2008) Three-dimensional cellular microarray for high-throughput toxicology assays. Proc Natl Acad Sci USA 105:59–63
Lein P, Locke P, Goldberg A (2007) Meeting report: alternatives for developmental neurotoxicity testing. Environ Health Perspect 115:764–768
Lewis RW, Billington R, Debryune E, Gamer A, Lang B, Carpanini F (2002) Recognition of adverse and nonadverse effects in toxicity studies. Toxicol Pathol 30:66–74
Li W, Ding S (2010) Small molecules that modulate embryonic stem cell fate and somatic cell reprogramming. Trends Pharmacol Sci 31:36–45
Loebel DA, Watson CM, De Young RA, Tam PP (2003) Lineage choice and differentiation in mouse embryos and embryonic stem cells. Dev Biol 264:1–14
Lu J, Helsby N, Palmer BD, Tingle M, Baguley BC, Kestell P, Ching LM (2004) Metabolism of thalidomide in liver microsomes of mice, rabbits, and humans. J Pharmacol Exp Ther 310:571–577
Luch A, Kishiyama S, Seidel A, Doehmer J, Greim H, Baird WM (1999a) The K-region trans-8, 9-diol does not significantly contribute as an intermediate in the metabolic activation of dibenzo[a, l]pyrene to DNA-binding metabolites by human cytochrome P450 1A1 or 1B1. Cancer Res 59:4603–4609
Luch A, Schober W, Soballa VJ, Raab G, Greim H, Jacob J, Doehmer J, Seidel A (1999b) Metabolic activation of dibenzo[a, l]pyrene by human cytochrome P450 1A1 and P450 1B1 expressed in V79 Chinese hamster cells. Chem Res Toxicol 12:353–364
Luciano AM, Franciosi F, Lodde V, Corbani D, Lazzari G, Crotti G, Galli C, Pellizzer C, Bremer S, Weimer M, Modina SC (2010) Transferability and inter-laboratory variability assessment of the in vitro bovine oocyte maturation (IVM) test within ReProTect. Reprod Toxicol 30:81–88
Maitra A, Arking DE, Shivapurkar N, Ikeda M, Stastny V, Kassauei K, Sui G, Cutler DJ, Liu Y, Brimble SN, Noaksson K, Hyllner J, Schulz TC, Zeng X, Freed WJ, Crook J, Abraham S, Colman A, Sartipy P, Matsui S, Carpenter M, Gazdar AF, Rao M, Chakravarti A (2005) Genomic alterations in cultured human embryonic stem cells. Nat Genet 37:1099–1103
Maltsev VA, Rohwedel J, Hescheler J, Wobus AM (1993) Embryonic stem cells differentiate in vitro into cardiomyocytes representing sinusnodal, atrial and ventricular cell types. Mech Dev 44:41–50
Marx-Stoelting P, Adriaens E, Ahr HJ, Bremer S, Garthoff B, Gelbke HP, Piersma A, Pellizzer C, Reuter U, Rogiers V, Schenk B, Schwengberg S, Seiler A, Spielmann H, Steemans M, Stedman DB, Vanparys P, Vericat JA, Verwei M, van der Water F, Weimer M, Schwarz M (2009) A review of the implementation of the embryonic stem cell test (EST). The report and recommendations of an ECVAM/ReProTect workshop. Altern Lab Anim 37:313–328
Massague J, Blain SW, Lo RS (2000) TGFβ signaling in growth control, cancer, and heritable disorders. Cell 103:295–309
Mazzoleni G, di Lorenzo D, Steimberg N (2009) Modelling tissues in 3D: the next future of pharmaco-toxicology and food research? Genes Nutr 4:13–22
Ming JE, Roessler E, Muenke M (1998) Human developmental disorders and the Sonic hedgehog pathway. Mol Med Today 4:343–349
Moeller HC, Mian MK, Shrivastava S, Chung BG, Khademhosseini A (2008) A microwell array system for stem cell culture. Biomaterials 29:752–763
Moller A, Soldan M, Völker U, Maser E (2001) Two-dimensional gel electrophoresis: a powerful method to elucidate cellular responses to toxic compounds. Toxicology 160:129–138
Murry CE, Keller G (2008) Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132:661–680
Nagel R (2002) DarT: the embryo test with the Zebrafish Danio rerio—a general model in ecotoxicology and toxicology. ALTEX 19:38–48
Najm FJ, Chenoweth JG, Anderson PD, Nadeau JH, Redline RW, McKay RD, Tesar PJ (2011) Isolation of epiblast stem cells from preimplantation mouse embryos. Cell Stem Cell 8:318–325
National Research Council (2007) Toxicity testing in the 21st century: a vision and a strategy, The National Academies Press, Washington DC
Nau H (2001) Teratogenicity of isotretinoin revisited: species variation and the role of all-trans-retinoic acid. J Am Acad Dermatol 45:S183–S187
Nirmalanandhan VS, Sittampalam GS (2009) Stem cells in drug discovery, tissue engineering, and regenerative medicine: emerging opportunities and challenges. J Biomol Screen 14:755–768
OECD (2001) Two-generation reproduction toxicity study, guidelines for the testing of chemicals TG 416, Organisation for Economic Cooperation and Development, Paris
Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA (2008) Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol 26:313–315
Pampaloni F, Stelzer EH, Masotti A (2009) Three-dimensional tissue models for drug discovery and toxicology. Recent Pat Biotechnol 3:103–117
Paquette JA, Kumpf SW, Streck RD, Thomson JJ, Chapin RE, Stedman DB (2008) Assessment of the embryonic stem cell test and application and use in the pharmaceutical industry. Birth Defects Res B Dev Reprod Toxicol 83:104–111
Pessina A, Albella B, Bayo M, Bueren J, Brantom P, Casati S, Croera C, Gagliardi G, Foti P, Parchment R, Parent-Massin D, Schoeters G, Sibiril Y, van den Heuvel R, Gribaldo L (2003) Application of the CFU-GM assay to predict acute drug-induced neutropenia: an international blind trial to validate a prediction model for the maximum tolerated dose (MTD) of myelosuppressive xenobiotics. Toxicol Sci 75:355–367
Peters AK, Steemans M, Hansen E, Mesens N, Verheyen GR, Vanparys P (2008a) Evaluation of the embryotoxic potency of compounds in a newly revised high throughput embryonic stem cell test. Toxicol Sci 105:342–350
Peters AK, Wouwer GV, Weyn B, Verheyen GR, Vanparys P, Gompel JV (2008b) Automated analysis of contractility in the embryonic stem cell test, a novel approach to assess embryotoxicity. Toxicol In Vitro 22:1948–1956
Phillippi JA, Miller E, Weiss L, Huard J, Waggoner A, Campbell P (2008) Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle- and bone-like subpopulations. Stem Cells 26:127–134
Phillips BW, Crook JM (2010) Pluripotent human stem cells: a novel tool in drug discovery. BioDrugs 24:99–108
Quigley D, Simmons F, Whyte H, Robertson J, Freshwater D (2010) Variations in reproductive and developmental toxicant identification. J Chem Health Saf 17:29–53
Rovida C, Hartung T (2009) Re-evaluation of animal numbers and costs for in vivo tests to accomplish REACH legislation requirements for chemicals—a report by the transatlantic think tank for toxicology t(4). ALTEX 26:187–208
Russell WMS, Burch RL (1959) The principles of humane experimental technique. Methuen, London
Sachinidis A, Fleischmann BK, Kolossov E, Wartenberg M, Sauer H, Hescheler J (2003) Cardiac specific differentiation of mouse embryonic stem cells. Cardiovasc Res 58:278–291
Sadler TW (2000) Susceptible periods during embryogenesis of the heart and endocrine glands. Environ Health Perspect 108:555–561
Schaafsma G, Kroese ED, Tielemans EL, van de Sandt JJ, van Leeuwen CJ (2009) REACH, non-testing approaches and the urgent need for a change in mind set. Regul Toxicol Pharmacol 53:70–80
Schaefer WR, Fischer L, Deppert WR, Hanjalic-Beck A, Seebacher L, Weimer M, Zahradnik HP (2010) In vitro-Ishikawa cell test for assessing tissue-specific chemical effects on human endometrium. Reprod Toxicol 30:89–93
Schardein JL, Keller KA (1989) Potential human developmental toxicants and the role of animal testing in their identification and characterization. Crit Rev Toxicol 19:251–339
Schenk B, Weimer M, Bremer S, van der Burg B, Cortvrindt R, Freyberger A, Lazzari G, Pellizzer C, Piersma A, Schäfer WR, Seiler A, Witters H, Schwarz M (2010) The ReProTect feasibility study, a novel comprehensive in vitro approach to detect reproductive toxicants. Reprod Toxicol 30:200–218
Schneider MR, Wolf E, Braun J, Kolb HJ, Adler H (2008) Canine embryo-derived stem cells and models for human diseases. Hum Mol Genet 17:R42–R47
Seiler A, Spielmann H (2011) The validated embryonic stem cell test to predict embryotoxicity in vitro. Nat Protoc 6:961–978
Seiler A, Visan A, Buesen R, Genschow E, Spielmann H (2004) Improvement of an in vitro stem cell assay for developmental toxicity: the use of molecular endpoints in the embryonic stem cell test. Reprod Toxicol 18:231–240
Seiler A, Buesen R, Visan A, Spielmann H (2006) Use of embryonic stem cells in embyotoxicity assays. Methods Mol Biol 329:371–395
Seiler A, Baumann W, Bicker G, Fritsche E, Genschow E, Gimsa J, Hayess K, Kaufmann W, Klemm M, Schrattenholz A (2007) The new BMBF joint project on the development of predictive in vitro tests for developmental neurotoxicity testing. ALTEX 24:225
Snykers S, De Kock J, Rogiers V, Vanhaecke T (2009) In vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem Cells 27:577–605
Soen Y, Mori A, Palmer TD, Brown PO (2006) Exploring the regulation of human neural precursor cell differentiation using arrays of signaling microenvironments. Mol Syst Biol 2:37
Spielmann H (2009) The way forward in reproductive/developmental toxicity testing. Altern Lab Anim 37:641–656
Spielmann H, Vogel R (2007) The extended 1-generation study (OECD 416), as a replacement of the mammalian 2-generation study (OECD 415). AATEX 14:795–798
Spielmann H, Pohl I, Döring B, Liebsch M, Moldenhauer F (1997) The embryonic stem cell test (EST), an in vitro embyotoxicity test using two permanent mouse cell lines: 3T3 fibroblasts and embryonic stem cells. In Vitro Mol Toxicol J Basic Appl Res 10:119–127
Surani MA, Hayashi K, Hajkova P (2007) Genetic and epigenetic regulators of pluripotency. Cell 128:747–762
Tanaka TS, Davey RE, Lan Q, Zandstra PW, Stanford WL (2008) Development of a gene-trap vector with a highly sensitive fluorescent protein reporter system for expression profiling. Genesis 46:347–356
Taylor K, Gordon N, Langley G, Higgins W (2008) Estimates for worldwide laboratory animal use in 2005. Altern Lab Anim 36:327–342
The European Chemical Industry Council (2010) Facts and figures—the European chemical industry in a worldwide perspective. CEFIC, Brussels
Trosko JE (2010) Commentary on ‘‘Toxicity testing in the 21st century: A vision and a strategy’’: stem cells and cell–cell communication as fundamental targets in assessing the potential toxicity of chemicals. Hum Exp Toxicol 29:21–29
Trosko JE, Chang CC (2010) Factors to consider in the use of stem cells for pharmaceutic drug development and for chemical safety assessment. Toxicology 270:18–34
Uibel F, Mühleisen A, Köhle C, Weimer M, Stummann TC, Bremer S, Schwarz M (2010) ReProGlo: a new stem cell-based reporter assay aimed to predict embryotoxic potential of drugs and chemicals. Reprod Toxicol 30:103–112
Vaags AK, Rosic-Kablar S, Gartley CJ, Zheng YZ, Chesney A, Villagómez DA, Kruth SA, Hough MR (2009) Derivation and characterization of canine embryonic stem cell lines with in vitro and in vivo differentiation potential. Stem Cells 27:329–340
van der Jagt K, Munn S, Tørsløv J, de Bruijn J (2004) Alternative approaches can reduce the use of test animals under REACH, Report EUR 21405 EN, European Commission Joint Research Centre
Vojnits K, Bremer S (2010) Challenges of using pluripotent stem cells for safety assessments of substances. Toxicology 270:10–17
Voutchkova AM, Osimitz TG, Anastas PT (2010) Toward a comprehensive molecular design framework for reduced hazard. Chem Rev 110:5845–8582
Walker E, Ohishi M, Davey RE, Zhang W, Cassar PA, Tanaka TS, Der SD, Morris Q, Hughes TR, Zandstra PW, Stanford WL (2007) Prediction and testing of novel transcriptional networks regulating embryonic stem cell self-renewal and commitment. Cell Stem Cell 1:71–86
Wang S, Tang X, Niu Y, Chen H, Li B, Li T, Zhang X, Hu Z, Zhou Q, Ji W (2007) Generation and characterization of rabbit embryonic stem cells. Stem Cells 25:481–489
Whitlow S, Bürgin H, Clemann N (2007) The embryonic stem cell test for the early selection of pharmaceutical compounds. ALTEX 24:3–7
Winkler J, Sotiriadou I, Chen S, Hescheler J, Sachinidis A (2009) The potential of embryonic stem cells combined with -omics technologies as model systems for toxicology. Curr Med Chem 16:4814–4827
Wobus A, Löser P (2011) Present state and future perspectives of using pluripotent stem cells in toxicology research. Arch Toxicol 85:79–117
Wobus AM, Wallukat G, Hescheler J (1991) Pluripotent mouse embryonic stem cells are able to differentiate into cardiomyocytes expressing chronotropic responses to adrenergic and cholinergic agents and Ca2+ channel blockers. Differentiation 48:173–182
Xia M, Huang R, Witt KL, Southall N, Fostel J, Cho M-H, Jadhav A, Smith CS, Inglese J, Portier CJ, Tice RR, Austin CP (2008) Compound cytotoxicity profiling using quantitative high-throughput screening. Environ Health Perspect 116:284–291
Yu J, Thomson JA (2008) Pluripotent stem cell lines. Genes Dev 22:1987–1997
Zhao X, Ruan Y, Wei CL (2008) Tackling the epigenome in the pluripotent stem cells. J Genet Genomics 35:403–412
zur Nieden NI, Baumgartner L (2010) Assessing developmental osteotoxicity of chlorides in the embryonic stem cell test. Reprod Toxicol 30:277–283
zur Nieden NI, Kempka G, Ahr HJ (2004) Molecular multiple endpoint embryonic stem cell test—a possible approach to test for the teratogenic potential of compounds. Toxicol Appl Pharmacol 194:257–269
zur Nieden NI, Davis LA, Rancourt DE (2010) Comparing three novel endpoints for developmental osteotoxicity in the embryonic stem cell test. Toxicol Appl Pharmacol 247:91–97
Author information
Authors and Affiliations
Corresponding author
Additional information
Andrea Seiler, Michael Oelgeschläger, Manfred Liebsch, Ralph Pirow, Christian Riebeling, Tewes Tralau and Andreas Luch contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Seiler, A., Oelgeschläger, M., Liebsch, M. et al. Developmental toxicity testing in the 21st century: the sword of Damocles shattered by embryonic stem cell assays?. Arch Toxicol 85, 1361–1372 (2011). https://doi.org/10.1007/s00204-011-0767-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-011-0767-1