Abstract
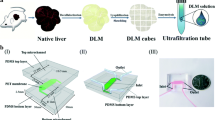
Proof of principle of organ reengineering through the development of a transplantable recellularized liver graft was published recently. As the decellularization time of the rat liver took 72 h, loss of some key matrix proteins seemed inevitable. Here, we describe the development of a three-dimensional naturally derived liver scaffold with an intact microvascular system that is capable of withstanding fluid flows in the three hepatic circular systems and that is obtained within 60 min. For this purpose, whole rat livers were sequentially perfused with a selection of mild tensioactive substances to remove the cellular components while preserving the major extracellular matrix proteins, including laminin, collagen I, collagen IV, and fibronectin. In addition, we could show the presence of extracellular matrix–bound growth factor islets, important for cell engraftment, migration, proliferation, and differentiation. This easy to prepare scaffold could represent a remarkable tool in the bioengineering of complex three-dimensional in vitro systems for advanced preclinical drug development.




Similar content being viewed by others
Abbreviations
- DLM:
-
Decellularized liver matrix
- ECM:
-
Extracellular matrix
- KHB:
-
Krebs-Henseleit buffer
- PBS:
-
Phosphate-buffered saline
- SEM:
-
Scanning electron microscopy
- SDS:
-
Sodium dodecyl sulfate
- 3D:
-
Three-dimensional
- VEGF:
-
Vascular endothelial growth factor
References
Badylak SF (2007) The extracellular matrix as a biologic scaffold material. Biomaterials 28(25):3587–3593
De Spiegelaere W, Casteleyn C, Van Den Broeck W, Simoens P (2008) Electron microscopic study of the porcine choroid plexus epithelium. Anat Histol Embryol 37:458–463
De Spiegelaere W, Cornillie P, Casteleyn C, Burvenich C, Van den Broeck W (2010) Detection of hypoxia inducible factors and angiogenic growth factors during foetal endochondral and intramembranous ossification. Anat Histol Embryol 39:376–384
Gómez-Lechón MJ, Lahoz A, Gombau L, Castell JV, Donato MT (2010) In vitro evaluation of potential hepatotoxicity induced by drugs. Curr Pharm Des 16(17):1963–1977
Guguen-Guillouzo C, Guillouzo A (2010) General review on in vitro hepatocyte models and their applications. Methods Mol Biol 640:1–40
Henkens T, Vanhaecke T, Papeleu P, Elaut G, Vinken M, Snykers S, Rogiers V (2006) Rat hepatocyte cultures: conventional monolayer and cocultures with rat liver epithelial cells. Methods Mol Biol 320:239–246
Järveläinen H, Sainio A, Koulu M, Wight TN, Penttinen R (2009) Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev 61(2):198–223
Papeleu P, Vanhaecke T, Henkens T, Elaut G, Vinken M, Snykers S, Rogiers V (2006) Isolation of rat hepatocytes. Methods Mol Biol 320: 229–237
Park JE, Keller GA, Ferrara N (1993) The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell 4(12):1317–1326
Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K (2010) Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 16(7):814–820
Vinken M, Elaut G, Henkens T, Papeleu P, Snykers S, Vanhaecke T, Rogiers V (2006) Rat hepatocyte cultures: collagen gel sandwich and immobilization cultures. Methods Mol Biol 320:247–254
Wang S, Nagrath D, Chen PC, Berthiaume F, Yarmush ML (2008) Three-dimensional primary hepatocyte culture in synthetic self-assembling peptide hydrogel. Tissue Eng 14(2):227–236
Acknowledgments
The authors thank Tatyana Doktorova, Jurgen De Craene, and Godelieve De Pauw for their excellent technical assistance.
Financial support
Joery De Kock is a doctoral research fellow of the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen). The research leading to these results has also received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement n°20161 (ESNATS) and from ISRIB (Brustem) and BELSPO (IAP).
Conflict of interest
The authors state that no conflict of interest is applicable to the presented work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Kock, J., Ceelen, L., De Spiegelaere, W. et al. Simple and quick method for whole-liver decellularization: a novel in vitro three-dimensional bioengineering tool?. Arch Toxicol 85, 607–612 (2011). https://doi.org/10.1007/s00204-011-0706-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-011-0706-1