Abstract
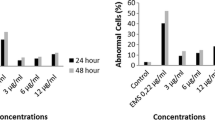
In this study, the effect of environmental toxins such as pentachlorophenol (PCP), tetrachlorocatechol (TeCC) and tetrachloroguaiacol (TeCG) on human peripheral blood lymphocytes was investigated. All the compounds studied increased the size and granularity of the lymphocytes in the concentrations range from 5 to 600 ppm. The PCP caused the strongest increase in the size of the cells, whereas lymphocytes granularity was more strongly increased by TeCC and PCP than by TeCG. The PCP and its derivatives in the concentrations range from 1 to 125 ppm significantly depleted ATP level. It was also observed that PCP most strongly decreased ATP content at its highest concentration of 125 ppm. Moreover, PCP caused the highest loss of lymphocytes viability in the concentrations range from 125 to 600 ppm. The TeCC in the concentrations of 1 and 5 ppm significantly increased the level of strand breaks in DNA, whereas lower damage was noted for PCP, and particularly for TeCG. The increase in carbonyl groups content was more strongly induced by TeCG and TeCC than by PCP in the concentrations range from 0.04 to 1 ppm; however, in a concentration of 5 ppm, all the compounds studied increased this parameter to a similar degree. DNA and protein damage was the most probably induced by free radical formation, as it was observed that all the compounds examined, and TeCC, in particular, were able of oxidize a fluorescent probe 6-carboxy-2′,7′-dichlorodihydrofluorescein in the concentrations range from 0.01 to 1 ppm.







Similar content being viewed by others
References
Andersson M, Hellman B (2007) Evaluation of catechol-induced DNA damage in human lymphocytes: a comparison between freshly isolated lymphocytes and T-lymphocytes from extended cultures. Toxicol In Vitro 21:716–722
Asmus KD (1990) Sulfur-centered free radicals. Methods Enzymol 186:168–180
ATSDR (2001) Toxicological profile for pentachlorophenol. Agency for Toxic Substances and Diesase Registry, Public Health Service, U.S. Department of Health and Human Services, Atlanta, p 316
Barstad A, Peyton D, Smejtek P (1993) AHA-heterodimer of class-2 uncoupler: pentachlorophenol. Biochim Biophys Acta 1140:262–270
Bartosz G (2006) Use of spectroscopic probes for detection of reactive oxygen species. Clin Chim Acta 368:53–76
Becker K, Schultz C, Kaus S, Seiwert M, Seifert B (2003) German environmental survey 1998 (Ger ES III): environmental pollutants in urine of the German population. Int J Hyg Environ Health 206:15–24
Berlett B, Stadtman E (1997) Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272:20313–20316
Brodeur J, Dixon G, Mckinley S (2001) Inhibition of oxygen consumption by pentachlorophenol and tetrachloroguaiacol in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 54:143–151
Bukowska B, Michałowicz J, Krokosz A, Sicińska P (2007) Comparison of the effect of phenol and its derivatives on protein and free radical formation in human erythrocytes (in vitro). Blood Cells Mol Dis 39:238–244
Carizo D, Grimalt J, Ribas-Fito N, Torrent M, Sunyer J (2008) Pentachlorobenzene, hexachlorobenzene and pentachlorophenol in children’s serum from industrial and rural populations after restricted use. Ecotoxicol Environ Saf 71:260–266
Carstens C, Blum J, Wittte I (1990) The role of hydroxyl radical in tetrachloroquinone induced DNA strand breaks formation in PM2 DNA and human fibroblasts. Chem Biol Interact 74:305–314
Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Marksesbery WR, Butterfield DA (2002) Proteomic identification of oxidatively modificated proteins in Alzheimer disease brain. Part I. Creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med 33:562–571
Catallo W, Shupe T (2008) Hydrothermal treatment of mixed preservative-treated wood waste. Holzforshung 62:119–122
Chhabra R, Maronot R, Bucher J, Haseman J, Toft J, Hejtmancik M (1999) Toxicology and carcinogenesis studies of pentachlorophenol in rats. Toxicol Sci 48:14–20
Cline R, Hill RH, Philips D, Needham L (1989) Pentachlorophenol measurements in body fluids of people in log homes and workplaces. Arch Environ Health 18:475–478
Crosby D, Beynon K, Greve P, Korte F, Still G, Vouk J (1981) Environmental chemistry of pentachlorophenol. Pure Appl Chem 53:1051–1080
Daniel V, Huber W, Bauer K, Opelz G (1997) Impaired in vitro lymphocyte responses in patients with elevated pentachlorophenol (PCP) blood levels. Arch Environ Health 50:148–149
Daniel V, Huber W, Bauer K, Suesal C, Condradt C, Opeltz G (2001) Association of blood levels of PCP, HCHs and HCB with numbers of lymphocyte subpopulations in vitro lymphocyte response plasma cytokine levels and immunoglobulin autoantibodies. Environ Health Perspect 109:173–178
Davies M, Fu S, Wang H, Dean T (1999) Stable markers of oxidant damage to proteins and their application in the study of human disease. Free Radic Biol Med 27:1151–1163
De Grooth B, Terstappen L, Pupples G, Greve J (1987) Light-scattering polarization measurements as a new parameter in flow cytometry. Cytometry 8:539–547
Dean R, Shanlin F, Stocker R, Davies M (1997) Biochemistry and pathology of radical-mediated protein oxidation. Biochem J 324:1–18
Efemrova S, Itskovich B, Parfenova V, Drucker V, Muller W, Schroder C (2002) Lake Baikal: a unique place to study evolution of sponges and their stress response in an environment nearly unimpaired by anthropogenic perturbation. Cell Mol Biol 48:359–371
Goldman R, Claycamp GH, Sweetland MA, Sedlow AV, Tyurin VA, Kisin ER, Tyurina YY, Ritov VB, Wenger SL, Grant GS, Kagan VE (1999) Myeloperoxidase-catalyaed redox-cycling of phenol promotes lipid peroxidation and thiol oxidation in HL-60 cells. Free Radic Biol Med 27:1050–1063
Grimaldi MCh, Hicks R, Diamond B (2005) Cell selection and susceptibility to autoimmunity. J Immunol 174:1775–1781
Jansson T, Curvall H, Hedin A, Enzel C (1986) In vitro studies of biological effects of cigarette smoke condensate. Induction of sister-chromatid exchanges in human lymphocytes by weakly acids semivolatile constituents. Mut Res 169:129–136
Jiang J, Chen J, Yu H, Zhang F, Zhang J, Wang L (2004) Quantitative structure activity relationship and toxicity mechanisms of chlorophenols on cells in vitro. Chin Sci Bull 49:562–566
Jorens PG, Schepens PJ (1993) Human pentachlorophenol poisoning. Hum Exp Toxicol 12:479–495
Jung J, Ishida K, Nishihara T (2004) Anti-estrogenic activity of fifty chemicals evaluated by in vitro assays. Life Sci 74:3065–3074
Levine R, Garland C, Oliver A, Amici A, Clinrent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman E (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Lown J (1985) Molecular mechanisms of action of anticancer agents involving free radical intermediates. Adv Free Radic Biol Med 1:225–264
Lui H, Sweeney G (1975) Hepatic metabolism of hexachlorobenzene in rats. FEBS Lett 51:225–226
Lunte SM, Kissinger PT (1983) Detection and identification of sulphydryl conjugates of p-benzoquinone in microsomal incubations of benzene and phenol. Chem Biol Interact 47:195–212
McConnell L, Bidleman T (1998) Collection of two-ring aromatic hydrocarbons, chlorinated phenols, guaiacols and benzenes from ambient air using polyurethane foam/tenax-GC cartridgers. Chemosphere 37:885–898
Michałowicz J (2005) The occurrence of chlorophenols, chlorocatechols and chlorinated methoxyphenols in drinking water of the largest cities of Poland. Pol J Environ Stud 14:143–148
Michałowicz J, Duda W (2007) Phenols–sources and toxicity. Pol J Environ Stud 16:347–362
Naito S, Ono Y, Somiva I, Inoune S, Ito K, Yamamoto K, Kawanishi S (1994) Role of active oxygen species n DNA damage by PCP metabolites. Mut Res 310:79–88
Nappi A, Vass E (1997) Comparative studies of enhanced iron-mediated production of hydroxyl radical by glutathione, cysteine, ascorbic acid and selected catechols. Biochim Biophys Acta 1336:295–301
Nikotera P, Leist M, Ferrnando-May E (1998) Intracellular ATP, a switch in the decision between apoptosis and necrosis. Toxicol Let 102–103:139–142
Oikari A, Walden R, Pritchard J (1995) Inhibition of renal xenobiotic excretion by tetrachloroguaiacol: mechanism and possible consequences. Environ Toxicol Chem 14:669–677
Ozaki A, Yamaguchi Y, Fujita T, Kuroda K, Endo G (2004) Chemical analysis and genotoxic safety assessment of paper and paperboard used for ford packaging. Food Chem Toxicol 42:1323–1337
Papadopoulos N, Dedoussis G, Spanakos G, Gritzapis A, Baxevanis C, Papamichail M (1994) An improved fluorescence assay for determination of lymphocyte-mediated cytotoxicity using flow cytometry. J Immunol Methods 177:101–109
Renner G, Hopper C (1990) Metabolic studies on pentachlorophenol (PCP) in rats. Xenobiotica 20:573–582
Rogers I, Levings C, Lockhart W, Norstrom R (1989) Observation of overwintering juvenile Chinook salmon (Oncorhynchus tshawutscha) exposed to bleached kraft mill effluent in Upper Fraser River, British Columbia. Chemosphere 19:1853–1868
Schultz C, Butte W (2007) Revised reference value for pentachlorophenol in morning urine. Int J Hyg Environ Health 44:741–744
Segura-Aguilar J, Baez S, Widersten M, Welch J, Mannervick C (1997) Human class Mu glutathione transferases, in particular isoenzyme M2–2, catalyze detoxication of the dopamine metabolite aminochrome. J Biol Chem 272:5727–5731
Shapiro H (1995) Practical flow cytometry. Wiley, New York
Singh N, McCoy T, Tice R, Schneider E (1988) A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res 175:184–192
Stanly P, Williams S (1969) Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem 29:381–392
Stoyanovski D, Goldman R, Claycamp G, Kagan V (1995) Phenoxyl radical-induced thiol-dependent generation of reactive oxygen species: implications for benzene toxicity. Arch Biochem Biophys 317:315–323
US Environmental Protection Agency (1998) Final pulp and paper cluster rules federal register 63:18503–18751
Wagner S, Durand L, Inman L, Kiigemagi U, Deinzer M (1991) Residue of pentachlorophenol and other chlorinated contaminants in human tissues: analysis by electron capture gas chromatography and electron capture negative ion mass spectrometry. Arch Environ Contam Toxicol 21:596–606
Wang Y, Ho Y, Jeng J, Su H, Lee C (2000) Different cell death mechanisms and gene expression in human cells induced by pentachlorophenol and its major metabolite tetrachlorohydroquinone. Chem Biol Interact 128:173–188
Webster G, Bowles M, Karim M, Wood R, Pockley A (1995) Flow cytometric analysis of peripheral blood lymphocyte subset light scatter characteristic as a means of monitoring the development of rat small bowel allograft rejection. Clin Exp Immunol 100:536–542
Weinbach E (1954) The effect of pentachlorophenol on oxidative phosphorylation. J Biol Chem 210:545–550
Xu F, Bell S, Rao Z, Wong L (2007) Structure-activity correlations in pentachlorobenzene oxidation engineered cytochrome P450 (Cam.). Protein Eng Des Sel 20:473–480
Yang N-Ch, Ho W-M, Chen Y-H, Hu M-L (2002) A convenient one-step extraction of cellular ATP using boiling water for the luciferin-luciferase assay of ATP. Anal Biochem 306:323–327
Zeng Z, Heng Z, Liao Y, Zhao R (2001) Study on DNA damage induced by indirect mutagens using comet assay in testicular cells in vitro. Carinogen Teratogen Mutagen 13:4–7
Acknowledgments
The author thanks Prof. Janusz Błasiak (Head of Department of Molecular Genetics, University of Łódź, Poland) for letting him use Eclipse fluorescence microscope for comet assay. This work was partly funded from costs of statutory investigation admitted for Department of Environmental Pollution Biophysics in the year of 2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michałowicz, J. Pentachlorophenol and its derivatives induce oxidative damage and morphological changes in human lymphocytes (in vitro). Arch Toxicol 84, 379–387 (2010). https://doi.org/10.1007/s00204-010-0515-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-010-0515-y